Translate this page into:
Chronic Inflammation in Polycystic Ovarian Syndrome: Examining Biomarker–Driven Control Strategies to Reduce Population–Level Disease Burden

*Corresponding author: Abhijit G. Banerjee, Genomic Bio-Medicine Research and Incubation, Chhattisgarh, Off Maple Street, Dhanora, Durg, India abhijitb@cgbmri.co.in
-
Received: ,
Accepted: ,
How to cite this article: Banerjee A, Banerjee AG. Chronic inflammation in polycystic ovarian syndrome: Examining biomarker–driven control strategies to reduce population-level disease burden. Int J Transl Med Res Public Health. 2024;8:e013. doi: 10.25259/IJTMRPH_20_2024
Abstract
Background and Objective
Polycystic ovarian syndrome (PCOS) is a major public health concern in India, known to cause infertility, obesity, insulin resistance, and cardiovascular issues globally. Chronic inflammation, linked to the kynurenine pathway, is a key factor in PCOS. This review aims to identify inflammatory mediators and comorbid conditions that could be addressed through dietary or non-pharmacological interventions.
Methods
A comprehensive literature review was conducted using keywords such as PCOS, metabolic dysfunction, chronic inflammation, endocrine, immune response, and neuropsychiatric disorders. Databases searched included biomedical literature databases such as PubMed (NCBI), Scopus, Science Direct, and Google Scholar, utilizing Boolean operands. Out of 112 initial search results, a total of 29 articles were selected for analysis, supplemented by directed citation searches.
Results
Our review elucidates potential mechanisms of inflammation in PCOS and highlights how components of an anti-inflammatory diet may mitigate prevalent low-grade inflammation, thereby attenuating progression of the disease. Dietary supplements affecting gut health and physical fitness regimens also show promise in achieving these end goals.
Conclusion and Implications for Translation
Biomarkers of low-grade inflammation (hsCRP and IL-6), balance of biochemical metabolites of the kynurenine pathway (kynurenic acid vs. quinolinic acid) and balanced diet (anti-inflammatory) counseling might help reduce the impact of PCOS as a serious public health concern in adolescent and young women. Emphasizing biomarker-driven control strategies could help prevent the widespread prevalence of PCOS and reduce the burden on health systems in the South Asian population.
Keywords
Polycystic Ovarian Syndrome
Metabolic Diseases
Chronic Inflammation
Endocrine
Immune Response
Neuropsychiatric Disorders
![Graphical Abstract: Plausible mechanisms of PCOS and related metabolic dysfunctions leading to neurological and behavioral consequences. Adapted from the basic kynurenine pathway diagram [Jovanovic et al., 2020]. HAOO: 3-hydroxyanthranilate dioxygenase; IDO: Indoleamine 2,3-dioxygenase, KAT: Kynurenine aminotransferase, KMO: Kynurenine 3-monooxygenase, KYNU: Kynurenine kinase, PCOS: Polycystic ovarian syndrome, QPRT: Quinolinate phosphoribosyl transferase, TDO: Trp 2,3-dioxygenase. PCOS?: Whether the enzymatic conversion step catalyzed by KMO is responsible for progression of disease.](/content/160/2024/8/1/img/IJTMRPH-8-e013-g001.png)
-
Graphical Abstract: Plausible mechanisms of PCOS and related metabolic dysfunctions leading to neurological and behavioral consequences. Adapted from the basic kynurenine pathway diagram [Jovanovic et al., 2020]. HAOO: 3-hydroxyanthranilate dioxygenase; IDO: Indoleamine 2,3-dioxygenase, KAT: Kynurenine aminotransferase, KMO: Kynurenine 3-monooxygenase, KYNU: Kynurenine kinase, PCOS: Polycystic ovarian syndrome, QPRT: Quinolinate phosphoribosyl transferase, TDO: Trp 2,3-dioxygenase. PCOS?: Whether the enzymatic conversion step catalyzed by KMO is responsible for progression of disease.
INTRODUCTION
Background of the Study
In recent years, the role of low-grade inflammation and oxidative stress in the etiology of polycystic ovarian syndrome (PCOS) has received increased attention. Previous studies evaluating the effects of inflammatory mediators, oxidative stress, and endothelial dysfunction on ovarian function due to hyperandrogenism in patients with PCOS have shown the key role of these factors in the progression and development of disease complications.[1–6] Long-term exposure to PCOS has been associated with obesity, cardiovascular problems, and insulin resistance. Adipocytes within fat deposits secrete cytokine interleukin 6 (IL-6), which is a major inflammatory modulator in PCOS. PCOS patients have been shown to have elevated IL-6 concentrations, and NF-?B-activation induced other cytokines are directly associated with the control of IL-6.[7–10] A pilot investigation comparing the gut permeability, inflammatory state, and stool microbiome of women with PCOS and healthy controls revealed that the stool microbiome of PCOS patients was less diverse than that of healthy controls and was a distinct factor contributing to the rising global incidence of infertility in women with PCOS.[11] Metabolically, various dietary sources of energy driving inflammation, and particularly the kynurenine pathway involved in tryptophan metabolism in both mammals and its inhabitant gut microbiome, seems to be involved in the alteration of immune status as well as neurological disease in patients with PCOS.[12–16] A particular comparative study in Indian women population with varying dietary patterns showed most biomarkers of inflammation as raised in PCOS versus healthy controls.[13] Kynurenine metabolites play important roles in several human tissues, including immune cell modulation, giving rise to mitochondrial dysfunction and have dual roles, ranging from neuroprotection to neurotoxicity, eventually resulting in neurological disorders related symptoms associated with PCOS.[16–20] In addition, IL-6 as a major pro-inflammatory signaling pathway is known to have a role in several pathological conditions besides PCOS, including asthma, rheumatoid arthritis, cardiovascular diseases, and colon cancer [Figure 1].[14]
Objectives of the Study
We aimed to ascertain mediators of progression factors in PCOS that might be related due to chronic inflammation-causing lifestyles and/or dietary behaviors that could also be potentially affecting the mental health of subjects.
Scope
We hypothesized that certain “biological pathways and metabolites of such biochemical routes of synthesis, might be important in promotion of chronic inflammation, consequently resulting in progression of the PCOS disease.” Specific aims were to delineate the biomarkers significantly responsible for progression so as to be used to diagnose the PCOS early in adolescent females and thus help ameliorate through advisory nutritional counseling or non-pharmacological interventions in a way to address this growing public health concern.
METHODS
Search Strategy/Data Sources
Using the operators “AND” and “OR", the Science Direct, Scopus, NCBI, and Google Scholar databases were searched for related articles to review the current literature pertaining to time period between 2001 and 2023. The search strategy using MeSH terms was as follows: (“polycystic ovary syndrome” [MeSH Terms] OR PCOS [Text Word] AND metabolic [All Fields] OR immune [All Fields] OR (“endocrine” [MeSH Terms] AND (“inflammation” [MeSH Terms]) AND neurological [All Fields]).
Eligibility Criteria
Studies published from 2001 till 2023 in English language with full text in peer-reviewed journals (Scopus-empaneled) were included.
Inclusion Criteria
Articles containing the maximum number of MeSH search terms as depicted in the search strategy above were included in the study.
Exclusion Criteria
Articles not related to the pathological conditions, i.e., PCOS or not in English language, and abstracts were eliminated. Duplicates were removed.
Quality Assessment
Statistically valid results of the studies reviewed were verified for strength of association and sample size.
RESULTS
Initially, the search retrieved 112 documents [Figure 2]. After a thorough analysis of every study published on the topic (n = 23) and following general observational reporting methodology, a total of 29 articles were chosen for this review that also includes six cross-referenced articles from earlier studies identified through a directed citation search.
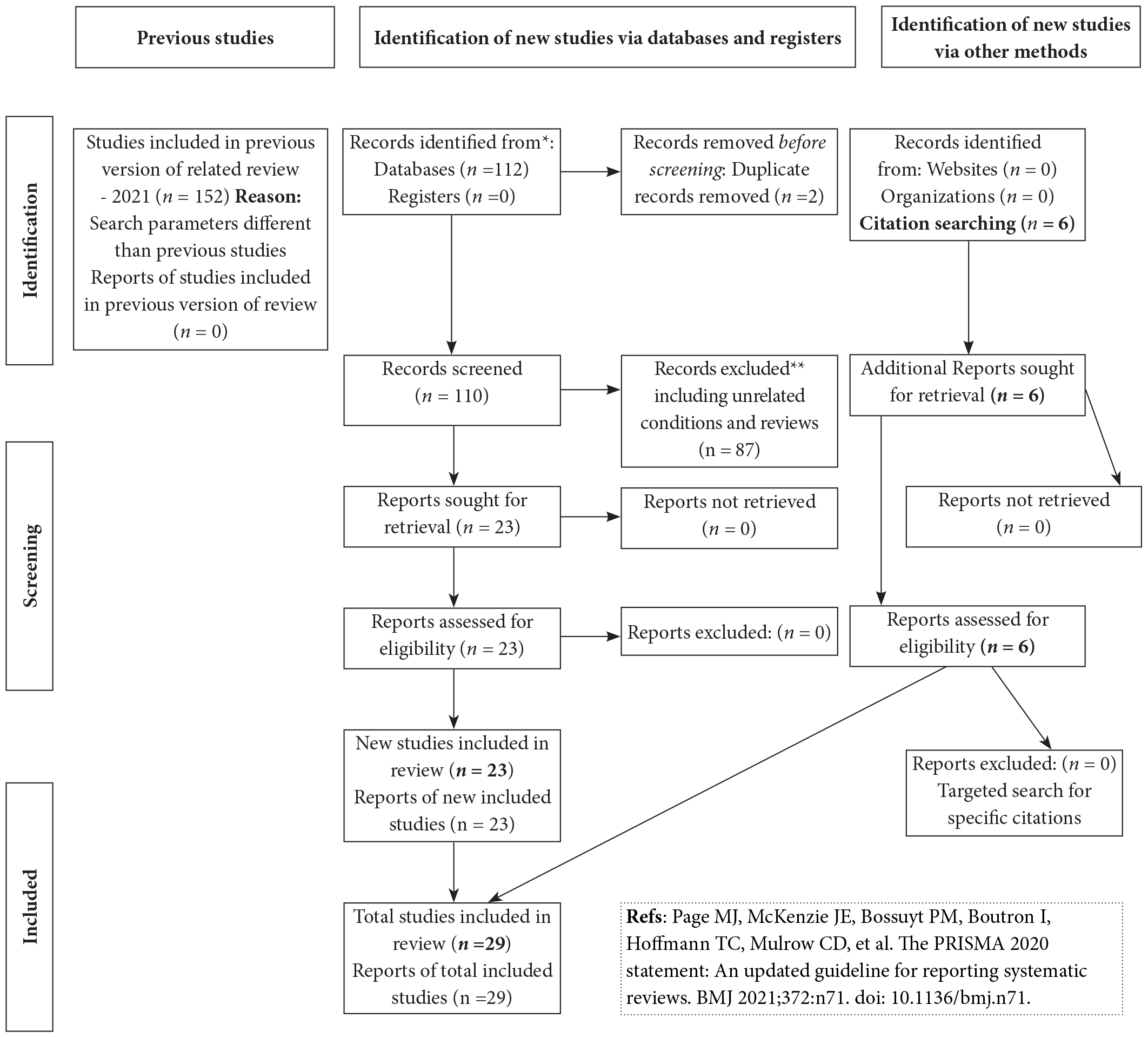
- PRISMA flow-chart diagram of the review work.
Overall, it is evident that low levels of inflammation termed also as “chronic inflammation” are linked to PCOS development, an immunological and hereditary disease that is crucial to the genesis, development, and incidence of the disease. Additionally, infertile PCOS patients receiving ART exhibit abnormal ovulatory activity, elevated oxygen free radical concentrations in granulosa cells, and elevated follicular fluid. It appears that the combination of three primary factors—high dietary carbohydrate intake, insulin resistance, and hyperandrogenism—could be responsible for oxidative stress and low-grade inflammation in PCOS patients.[12–17] Anti-inflammatory medications and antioxidant supplements may help these patients’ infertility treatment cycles improve, but ultimately, lifestyle changes are important.
DISCUSSION
The objective of the study was to delineate various biochemical factors responsible for creating long-term chronic, low-grade inflammation in the human body that can form the basis of early detection and prevention of PCOS. A pilot study in 2017 by Lindheim and colleagues compared the gut permeability, inflammatory conditions, and stool microbiome of women with PCOS with those of healthy controls. The study showed that patients with PCOS had a stool microbiome with reduced diversity and a different phylogenetic composition.[11] This added “gut health” as a third dimension besides low-grade inflammation and reproductive hormonal changes in women with PCOS. However, it remains to be deciphered what propels the progression of the disease in a sequential manner within gut-immune-brain axis of things, thus representing a critical knowledge gap. Further, in PCOS patients, the relative abundance of bacteria from the phylum Tenericutes, the order ML615J-28 within that phylum, and the family S24-7 within the phylum Bacteroidetes was much lower when uncommon taxa were examined.[11] Therefore, gut microbiota itself can now be used as a biomarker to detect disease early. Regarding overall gut microbiome composition, for any taxon with a relative abundance greater than 1%, no discernible changes were found. Although they did not cause clinical phenomena in the patient cohort, endotoxemia and gut barrier disruption might have contributed to the persistent low-grade inflammation identified in the pathophysiology of PCOS. Certain markers of endotoxemia and gut barrier function were altered in the patients, but not all of them. These findings were also linked to reproductive parameters. The authors concluded that the stool microbiome of PCOS patients has a changed phylogenetic profile and decreased diversity, which are linked to clinical characteristics. Independent of obesity, a dietary trigger such as glucose from carbohydrate dietary sources can cause oxidative stress and an inflammatory response in the mononuclear cells (MNCs) of women with PCOS.[10–13] This finding is significant because MNC-derived macrophages are the main source of cytokine production in adipose tissue and are abundant, and these cells stimulate cytokine production in adipocytes in a paracrine manner. TNFa, a proinflammatory cytokine, is recognized as an insulin resistance mediator. In PCOS, TNFa production from MNCs induced by glucose and inflammatory molecular markers is linked to insulin resistance. Under fasting conditions, hyperandrogenism can activate MNCs, which increases their sensitivity to glucose.[5] Inflammation and oxidative stress have been increasingly studied in the etiology of polycystic ovarian syndrome in recent years. The assessment of extant research concerning the impact of inflammatory mediators and oxidative stress on ovarian function in individuals diagnosed with PCOS highlights the pivotal function of these variables in the advancement and emergence of complications associated with the disease.[13] The goal of the current review was to provide an overview of the existence and function of inflammatory mediators as well as the impact of contemporary therapeutic modalities, such as the use of anti-inflammatory drugs and antioxidant supplements, on patients’ ability to respond better to infertility treatment cycles.
Increased C-reactive protein (CRP) concentrations, a sign of low-grade chronic inflammation, are an independent predictor of type 2 diabetes and coronary heart disease (CHD) risk. There are currently no data on the indicators of inflammation in women with polycystic ovarian syndrome (PCOS), even though these women are insulin resistant and at increased risk for type 2 diabetes and CHD.[7] Another pilot study involved 17 PCOS-afflicted women (identified by increased testosterone and oligomenorrhea) and 15 healthy women who were matched for body mass index. CRP levels were measured using an extremely sensitive technique. Insulin resistance was measured using the hyperinsulinemia euglycemic clamp technique. In comparison to those in controls, the CRP concentrations in PCOS-afflicted women were noticeably greater than those in healthy women (geometric means, 2.12 and 0.67 mg/L, respectively).[3]
PCOS patients have considerably higher levels of inflammatory factors in their peripheral blood, including human serum-CRP (hsCRP), IL-1R, IL-6, IL-17A, IL-17F, IL-18, IL-23, TNF-a, a-1 acid glycoprotein, monocyte chemoattractant protein-1 (MCP-1), adipokines, and their paralogs.[15] PCOS patients had significantly reduced levels of anti-inflammatory cytokines in their peripheral blood, including IL-10, IL-17E, IL-27, IL-35, IL-37, Omentin-1, and secreted frizzled-related protein 5 (SFRP5).[19] Furthermore, a pooled analysis of 63 studies found that PCOS women (n = 4,086) had substantially higher circulating CRP levels than controls (n = 3,120), with a standardized mean difference (SMD 1.26, 95% CI = 0.99, 1.53).[15] A sensitivity meta-analysis of 35 high-quality studies involving nonobese women found that PCOS patients had significantly higher circulating CRP levels than controls (SMD 1.80, 95% CI = 1.36, 2.25). The systemic inflammation underlying PCOS is thought to interact with comorbidities such as obesity, insulin resistance (IR), and hyperandrogenism. Ayurvedic and Traditional Chinese Medicine (TCM) are multitarget treatments for PCOS that involve anti-inflammatory and antioxidant agents and lifestyle modifications that can benefit women by alleviating inflammatory responses. However, the evidence for this link remains inconclusive, and its causal nature remains unclear.
A systematic review of studies investigating CRP and other inflammatory markers in PCOS patients versus healthy controls revealed significantly greater circulating CRP levels in PCOS patients than in controls.[15] A sensitivity meta-analysis of 35 high-quality studies including nonobese women showed significantly greater circulating CRP levels in PCOS patients than in controls. Thus, evidently, low-grade inflammation and inflammatory indicators are associated with PCOS.[21] Further, dietary modifications have been shown to significantly reduce inflammation and prevent disease progression.[21,22] Examining the inflammatory mediators and processes that lead to the onset and progression of PCOS can be a vital first step toward comprehending the pathophysiology of this rampant disease and helpful in developing strategies for treating it by blocking or regulating relevant signaling pathways. We address the pathophysiological roles of chronic low-grade inflammatory mediators, including cytokines linked to inflammasome formation, interleukin-1β (IL-1β), and IL-18, in PCOS development in the current review.
As per a WHO report, Metformin, the oldest oral antidiabetic drug, has been shown to improve the coexisting complications of diabetes, prevent or alleviate cardiovascular diseases, and ameliorate obesity, PCOS,[2] osteoporosis, cancer, periodontitis, neuronal damage and neurodegenerative diseases, inflammation, inflammatory bowel disease (IBD), tuberculosis, and even COVID-19. Therefore, it has been proposed to be an anti-aging “elixir” and has numerous mechanisms involved in its protective effects. However, there are long-term side effects and resistance phenomena to be wary of continuous therapy with metformin.
According to earlier researches,[23–25] oxidative stress and inflammation in the ovarian microenvironment might affect the physiological state of granulosa cells (GCs), which in turn can lead to abnormal follicular growth associated with PCOS. Thus, improving the ovarian microenvironment can be a practical way to boost the capacity for development of PCOS-related oocytes. GCs were obtained from control and PCOS patients to measure the expression of factors related to oxidative stress (HIF-1a and VEGFA) and inflammation (TGF-β1, IL-10, TNFa, and IL-6), as well as the ability of GCs to proliferate and increase the degree of apoptosis.[25] Next, the human ovarian granulosa cell line (KGN) was treated to confirm the anti-inflammatory and antioxidative stress properties of chitosan oligosaccharide (COS) so as to investigate the ideal culture duration.[25] Symptoms of polycystic ovarian morphology (PCOM), oligoanovulation, and hyperandrogenism are combined to cause PCOS. It is not confounding that the levels of certain inflammatory cytokines were always found elevated in PCOS, a condition marked by chronic inflammation. Hu et al. in 2023 found that increased expression of HMGB1, TLR2, and TLR4 proteins led to inflammation-related signaling pathways in the gravid uterus exposed to 5a-dihydrotestosterone and insulin. This mimicked the clinical features of PCOS, including hyperandrogenism and insulin resistance. Authors further delineate that elevated levels of HMGB1 and its receptors, as well as alteration of the pro-/anti-inflammatory balance in the gravid uterus, may contribute to the pathophysiology of PCOS-associated pregnancy loss.[26-29]
CONCLUSION AND IMPLICATIONS FOR TRANSLATION
In conclusion, while the precise mechanisms underlying inflammation in patients with PCOS remain unclear, obesity, insulin resistance, and elevated testosterone levels are believed to play significant roles in mediating this chronic inflammatory state. Persistent inflammatory conditions in the ovaries and uterus of PCOS patients adversely affect both the quality of life and the likelihood of developing additional health issues. Subsequently, developing strategies to reduce such inflammation albeit being low-grade, in adolescent and young women is crucial. Further, it may be concluded that circulating CRP is moderately but significantly elevated in PCOS women independent of obesity, which is indicative of low-grade, chronic inflammation and therefore stimulates the production of inflammatory cytokines, proteins that attract circulating monocytes, and other immune cell recruitment to elicit an inflammatory response that sustains the inflammatory state within adipocytes. Elevated circulating levels of hsCRP in PCOS women, independent of obesity, also suggest its potential as a biomarker for identifying low-grade chronic inflammation. Interventions targeting the kynurenine metabolic pathway, which feeds the patients’ health towards a low risk of chronic inflammation, may therefore mitigate and control PCOS progression. Hence, we draw following implications for translation and healthcare policy framework from the study:
Biomarkers such as CRP and IL6 and metabolites such as kynurenic acid to quinolinic acid ratio using tryptophan metabolite assays can serve as important analytes to detect PCOS early in order to identify kynurenine pathway dysfunction.
Recommendations for an anti-inflammatory diet and regular physical exercise present a potential non-pharmacological intervention strategy for susceptible individuals post-early diagnosis.
Key Messages
Polycystic ovarian syndrome is a complex lifestyle disorder involving endocrine, genetic, immune, metabolic and neurological systems.
Chronic inflammation triggered by a high-carbohydrate, pro-glycemic, pro-inflammatory diet might be one of the risk factors responsible for early progression.
Non-pharmacological, anti-inflammatory diet regimens promoting better gut health and exercise schedules through professional outreach campaigns may help reduce this public health problem in adolescents and young women.
Acknowledgments
None.
COMPLIANCE WITH ETHICAL STANDARDS
Conflicts of Interest
The authors declare no competing interests.
Financial Disclosure
AGB provides R&D consulting services to emerging bioentrepreneurs and mentors postgraduate researchers, independently without an affiliation.
Funding/Support
No funding was received for the study.
Ethics Approval
Institutional Review Board approval is not required.
Declaration of Patient Consent
Patient’s consent not required as there are no patients in this study.
Use of Artificial Intelligence (AI)-Assisted Technology for Manuscript Preparation
The authors confirm that there was no use of Artificial Intelligence (AI)-Assisted Technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
None.
REFERENCES
- Low grade chronic inflammation in women with polycystic ovarian syndrome. J Clin Endocrinol Metab.. 2001;86((6)):2453-5.
- [CrossRef] [PubMed] [Google Scholar]
- Metformin reduces serum C-reactive protein levels in women with polycystic ovary syndrome. J Clin Endocrinol Metab.. 2003;88((10)):4649-54.
- [CrossRef] [PubMed] [Google Scholar]
- Increased C-reactive protein levels in the polycystic ovary syndrome: A marker of cardiovascular disease. J Clin Endocrinol Metab.. 2004;89((5)):2160-5.
- [CrossRef] [PubMed] [Google Scholar]
- Endothelial dysfunction in young women with polycystic ovary syndrome: Relationship with insulin resistance and low-grade chronic inflammation. J Clin Endocrinol Metab.. 2004;89((11)):5592-6.
- [CrossRef] [PubMed] [Google Scholar]
- Reactive oxygen species-induced oxidative stress in the development of insulin resistance and hyperandrogenism in polycystic ovary syndrome. J Clin Endocrinol Metab.. 2006;91((1)):336-40.
- [CrossRef] [PubMed] [Google Scholar]
- Indices of low-grade chronic inflammation in polycystic ovary syndrome and the beneficial effect of metformin. Hum Reprod.. 2006;21((6)):1426-31.
- [CrossRef] [PubMed] [Google Scholar]
- Risk of metabolic complications in the new PCOS phenotypes based on the Rotterdam criteria. Fertil Steril.. 2007;88((5)):1389-95.
- [CrossRef] [PubMed] [Google Scholar]
- The role of low-grade inflammation in the polycystic ovary syndrome. Mol Cell Endocrinol.. 2011;335((1)):30-41.
- [CrossRef] [PubMed] [Google Scholar]
- IL-6 serum levels and production is related to an altered immune response in polycystic ovary syndrome girls with insulin resistance. Mediators Inflamm.. 2011;2011:389317.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Adipose tissue dysfunction, adipokines, and low-grade chronic inflammation in polycystic ovary syndrome. Reproduction.. 2015;149((5)):R219-27.
- [CrossRef] [PubMed] [Google Scholar]
- Alterations in gut microbiome composition and barrier function are associated with reproductive and metabolic defects in women with polycystic ovary syndrome (PCOS): A pilot study. PLoS One.. 2017;12((1)):e0168390.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Source and amount of carbohydrate in the diet and inflammation in women with polycystic ovary syndrome. Nutr Res Rev.. 2018;31((2)):291-301.
- [CrossRef] [PubMed] [Google Scholar]
- Comparative evaluation of biomarkers of inflammation among Indian women with polycystic ovary syndrome (PCOS) consuming vegetarian vs. non-vegetarian diet. Front Endocrinol (Lausanne).. 2019;10:699.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Role of IL-6 signaling in polycystic ovarian syndrome associated inflammation. J Reprod Immunol.. 2020;141:103155.
- [CrossRef] [PubMed] [Google Scholar]
- The role of chronic inflammation in polycystic ovarian syndrome: a systematic review and meta-analysis. Int J Mol Sci.. 2021;22((5)):2734.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Mitochondrial dysfunction and chronic inflammation in polycystic ovary syndrome. Int J Mol Sci.. 2021;22((8)):3923.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Oxidative stress and low-grade inflammation in polycystic ovary syndrome: Controversies and new insights. Int J Mol Sci.. 2021;22((4)):1667.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Chronic inflammation in polycystic ovary syndrome: A case-control study using multiple markers. Int J Reprod Biomed.. 2021;19((4)):313-20.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Systemic and ovarian inflammation in women with polycystic ovary syndrome. J Reprod Immunol.. 2022;151:103628.
- [CrossRef] [PubMed] [Google Scholar]
- The kynurenine pathway and polycystic ovary syndrome: inflammation as a common denominator. Int J Tryptophan Res.. 2022;15:11786469221099214.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The effect on inflammation of adherence to the Mediterranean diet in polycystic ovary syndrome. Curr Nutr Rep.. 2023;12:191-202.
- [CrossRef] [PubMed] [Google Scholar]
- Quercetin and polycystic ovary syndrome; inflammation, hormonal parameters and pregnancy outcome: A randomized clinical trial. Am J Reprod Immunol.. 2023;89((3)):e13644.
- [CrossRef] [PubMed] [Google Scholar]
- The role of inflammation and oxidative stress in the etiology of polycystic ovary syndrome: A review article. Iran J Obstet Gynecol Infertil.. 2023;25((11)):73-87.
- [Google Scholar]
- The interplay between androgens and the immune response in polycystic ovary syndrome. J Transl Med.. 2023;21((1)):259.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Chitosan oligosaccharide improves ovarian granulosa cells inflammation and oxidative stress in patients with polycystic ovary syndrome. Front Immunol.. 2023;14:1086232.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Regulatory mechanisms of HMGB1 and its receptors in polycystic ovary syndrome-driven gravid uterine inflammation. FEBS J.. 2023;290((7)):1874-1906.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Glucagon-like peptide-1 receptor agonists decrease hyperinsulinemia and hyperandrogenemia in dehydroepiandrosterone-induced polycystic ovary syndrome mice and are associated with mitigating inflammation and inducing browning of white adipose tissue. Biol Reprod.. 2023;108((6)):941-50.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Polycystic ovary syndrome and related inflammation in radiomics; relationship with patient outcome. Semin Cell Dev Biol.. 2024;154(Pt C):328-33.
- [CrossRef] [PubMed] [Google Scholar]
- Polycystic ovary syndrome: etiology, current management, and future therapeutics. J Clin Med.. 2023;12:1454.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]





