Translate this page into:
Profiles and Characteristics of Patients with Mild to Moderate COVID-19 Disease Phenotypes in a Teaching Hospital in Kano, Northern Nigeria
✉Corresponding author email: hamisu.salihu@gmail.com
Abstract
Background and Introduction:
COVID-19 has affected almost 180 million people around the world, causing the death of about 5 million persons, as of November 16, 2021. The disease presents with a plethora of pulmonary and extrapulmonary symptoms of varying severity. After an exhaustive review of the literature, we found no data on the mild and moderate COVID-19 disease phenotypes in Northern Nigeria. Our objective is to describe the clinical characteristics of non-severe COVID -19 disease phenotypes in Kano State.
Methods:
This is a retrospective cohort study at the COVID-19 Isolation Center of Muhammad Buhari Specialist Hospital Kano, Nigeria. We included all patients admitted from May 2020 to December 2020. Patients' medical records were assessed and evaluated to describe the clinical characteristics at presentation. We explored time to discharge between patients aged ≤ 50 years old versus those >50. We applied the Kaplan-Meier product-limit estimator to generate cumulative probabilities of discharge over time and used the Log-rank test to determine differences between the two age groups. We applied Cox Proportional Hazards to identify predictors of time to discharge among the patients in the study. The study variables comprised of time of viral clearance and time to discharge as outcome variables, while main exposure variables included, age, sex, occupation, mode of exposure, presence of co-morbidity, and duration of hospitalization.
Results:
A total of 187 COVID-19 patients were reviewed. The commonest symptoms were fever, breathing difficulty, and dry cough. There was no recorded death. Contact with a confirmed COVID-19 positive person was the source of infection in 167(89.3%) of patients. We noted faster time to viral clearance in patients on lopinavir compared to those on chloroquine (Log-rank test p-value = 0.048). There were no significant differences in time to discharge between younger (< 50 years) versus older patients (≥ 50 years) [24 days vs. 26 days respectively; Log-rank test p-value = 0.082]. Age, sex, and source of infection did not appear to be predictors of infection phenotype.
Conclusion and Implications for Translation:
The findings of this study have a bearing on the surveillance and diagnosis of COVID-19 in Nigeria. While the plethora of clinical features may not be limited to infection with the SARS-CoV-2 virus, healthcare practitioners should consider these symptom clusters in addition to cognate contact and travel history when confronted with a suspected COVID-19 infection.
Keywords
COVID-19
Phenotypes
Kaplan-Meier
Time-to-Discharge
Cox Proportional Hazards
Nigeria
Introduction
At the end of 2019, a new pneumonia-causing coronavirus was identified from Wuhan in China, and for the purpose of uniformity, the World Health Organization (WHO) named the disease Corona Virus Disease 2019 (COVID-19), also known as Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2). Accordingly, the WHO declared the COVID-19 to be a disease of public health emergency of global concern.1 As of November 16, 2021, the WHO reported 253,163,330 confirmed cases of COVID-19 across the globe, with 5,098,174 fatal outcomes. Of these, 6,186,377 COVID-19 cases were reported from Africa.2 Nigeria had reported 213,175 PCR-confirmed cases of the disease with 2,968 associated deaths as of November 16, 2021.3
The clinical phenotypes of COVID-19 vary from asymptomatic infection through mild to moderate disease with symptoms comprising a variable combination of fever, cough fatigue, body aches, sore throat, loss of taste and/or smell as well as a host of other symptoms.4 In the severe form of the disease, it may progress to an acute respiratory distress syndrome (ARDS), and/or multiple organ failure, which could lead to death.5 More precise details of the disease (phenotypes), especially with respect to some unique clinical characteristics, are still evolving across the globe. Indeed, diverse subtypes of the disease not only have unique epidemiological features but also dissimilar laboratory findings. In Nigeria, Erinoso et al., reported the clinical characteristics of hospitalized patients in a cohort of 632 patients in Lagos6 while Otuonye et al.7 described the clinical profiles of 154 COVID-19 patients from the same State. Additionally, Elimian et al. described the cumulative incidence and case fatality of COVID-19 in Nigeria using retrospective data.8
Nigeria has a number of international airports, with the busiest ones being Lagos, Abuja, and Kano international airports. These points of entry represent potential sources of new COVID-19 infections into the country. While a large proportion of visitors to Lagos came from the United States and United Kingdom, those arriving in Kano are largely from the Middle East and Asia. Consequently, it is plausible to have imported cases of SARS-CoV-2 virus having varying clinical presentation and virulence resulting in clinical phenotypes. While the Nigerian Center for Disease Control (NCDC) continuously provides data on the burden of COVID-19 for each state of the country, little is known about predictors and clinical trajectory of non-severe COVID-19 disease phenotypes outside South-Western Nigeria after an exhaustive literature search.
Therefore, this study analyzed clinical characteristics of non-severe COVID-19 disease phenotypes confirmed using real-time Reverse Transcriptase Polymerase Chain Reaction (RT- PCR) in Kano City, Kano State. Kano city is the largest commercial city in Northern Nigeria with a population of about four million people.9 We also explored potential risk factors associated with the duration of hospitalization in order to provide useful information on disease phenotypes. After an exhaustive literature search utilizing the appropriate keywords on search engines comprising PUBMED, Web of Science, and Google Scholar, we found this study to be the first from Northern Nigeria that describes COVID-19 clinical phenotypes.
Methods
Study Sites and Participants
Kano is the commercial and largest city of Northern Nigeria and has consistently attracted the migration of people from other parts of Nigeria and neighboring African countries. We conducted a retrospective study with ethical approval from the ethics committee of Kano State Ministry of Health, Nigeria (Approval Number: (MOH/Off/797/T.I/2030).
Kano state has designated Muhammad Buhari Specialist Hospital (MBSH), an ultra-modern tertiary hospital in Kano state, to provide care for non-severe COVID-19 cases while the Kwanan-Dawaki General Hospital was designated for severe COVID-19 case management. With a 200 bed capacity, MBSH has an annual patient load of 1,500, with a staff strength of 36 doctors, 20 Consultants, and 88 Nurses. MBSH provides a broad range of clinical care and laboratory services. The permanent staff of MBSH were not involved in the care of COVID-19 patients rather a new well-trained team of caregivers were assembled to take care of COVID-19 patients. The new team comprised of doctors, nurses, infection control officers, and other support staff who formed part of the COVID-19 Task Force clinical care team. The team lead (manager) is a physician from Ahmadu Bello University Zaria (ABU) while the clinical lead is a physician from Bayero University Kano (BUK). Clinical care staff provides 24/7 in-patient care with compliance to universal precautions and COVID-19 prevention control measures. All data on managed cases were reported to the Nigerian Center for Disease Control (NCDC) using NCDC tools. A new COVID-19 laboratory was established by 54 genes corporation in collaboration with the NCDC. Quality assurance (QA) was provided by the NCDC National Reference Laboratory Gaduwa, Abuja, Nigeria.
Data from case notes of 187 patients admitted into the Muhammad Buhari Specialist Hospital (MBSH) COVID-19 isolation center in Kano State were abstracted. These patients were admitted between May 2020 and December 2020 and tested positive for SAR-CoV-2 regardless of symptom status. The COVID-19 isolation center at MBSH is designated for managing patients with non-severe disease only. Those with severe disease are managed at Kwanan-Dawaki General Hospital. The definition was based on the NCDC guideline, which describes non-severe COVID-19 infection as: Fever < 38 °C; No difficulty in breathing; Presence or absence of cough; No underlying chronic diseases (e.g., heart, lung, asthma, and kidney diseases) and; Not requiring oxygen supplementation.
Clinical Care Protocol
All data on managed cases were reported to the NCDC using NCDC tools.10 The tools comprised of the updated checklist from District Hospitals for Sample Collection, nCoV-converted, specimen referral form for COVID-19 SARS- CoV-2 virus testing, case investigation form, and death investigation form for COVID-19, as well as contact tracing form. Samples were taken by trained infection officers. Patient's eligibility for admission was assessed by the trained staff of the COVID-19 task force. The task force decided on place of care on a case-by-case basis in addition to acceptance of referred patients. All patients were confirmed to be positive for SARS-CoV-2 infection using RT- PCR run on samples obtained from the throat and nasopharynx. A positive PCR test was the basis for admission into the isolation center. The test were repeated after a week in accordance with the clinical guidelines recommended by the Nigeria Center for Disease Control (NCDC).11,12 Clinical care was provided by physicians and nurses with support from the pharmacy and general laboratory staff. A consultant pulmonologist provided overall clinical care leadership while a general physician served as the isolation center manager. Overall supervision was provided by the NCDC and WHO staff through technical assistance offered during periodic visits. Patients were discharged based on a composite assessment of symptoms and a negative PCR result. Those that had persisting positive SARS-CoV-2 PCR tests were further evaluated based on clinical assessment. Clinical outcomes were monitored and recorded by the clinicians at the center. All study patients were placed on vitamin C, zinc, and azithromycin. At the discretion of the management team, patients were allocated to either Chloroquine 500 mg 12 hourly for seven days or lopinavir (ritonavir 400 mg plus ritonavir 100 mg) by mouth every 12 hours for 10 days. The MBSH isolation center was part of the WHO Solidarity Clinical Trial,and its protocol had allowed the use of these drugs.
Data Collection and Statistical Analysis
We collected data for each patient consisting of demographic and clinical attributes including age, sex, occupation, mode of viral exposure, duration of time between testing positive and admission, duration of hospitalization, presence of co-morbidity, and an array of other symptoms.
The study outcomes variables were (1) viral clearance; and (2) time to discharge while exposure variables included, age, sex, occupation, mode of exposure, presence of comorbidity, and duration of hospitalization.
We summarized normally distributed continuous variables as mean (standard deviation, SD), and for those deviating from normality, we utilized the median (interquartile range, IQR). The Pearson's Chi- square test was used to assess for the existence of bivariate associations between categorical variables (symptom status, and viral clearance). We then explored and compared time to discharge between patients aged ≤ 50 years old versus those >50. This was based on the local observation that the 50 years cut-off seems to be the tipping point that often predicts how age affects disease presentation. We observed those presenting at age 50 years and above had a higher likelihood of presenting with severe COVID-19 disease.
We applied the Kaplan-Meier product-limit estimator to generate cumulative probabilities of discharge over time and used the Log-rank test to determine differences between the two age groups. We used Cox Proportional Hazards modeling to identify adjusted predictors of time to discharge which generated hazards ratios (HR). We adjusted for age, sex, occupation, and mode of exposure. Statistical analyses were conducted with STATA version 16 (STATA, College Station, TX, USA). We considered a two-tailed p-value of 0.05 as statistically significant.
Results
Demographics
The median age of the patients was 33.0 years (IQR: 19-48), with individuals 21-40 years of age having the highest frequency (36.9%) (Figure 1). Most of the patients were male (n = 133 or 71.51%), and the median hospital length of stay was 10 days (IQR 8-18).
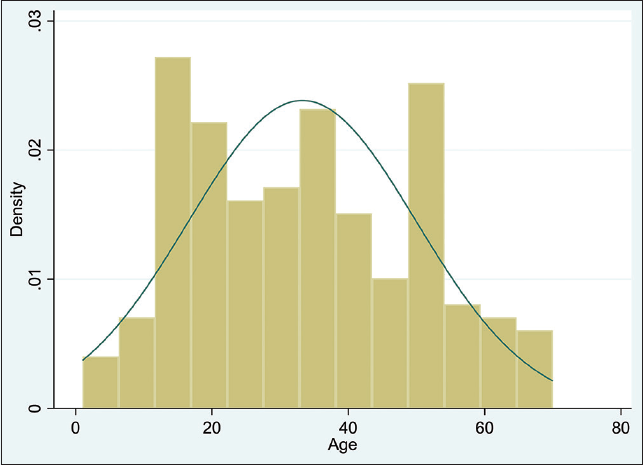
- Age distribution of the COVID-19 patients in Muhammad Buhari Isolation Center
Time of Test Confirmation to Admission
The mean time between testing positive and admission was 3 days, and contact with a confirmed COVID-19 positive person was the commonest mode of exposure (Table 1). We did not detect any significant differences between symptomatic and asymptomatic patients when we compared parameters such as age, sex, occupation, mode of exposure, presence of any co-morbidity, and duration of hospitalization (Table 2).
| Characteristic | Measure of Summary |
|---|---|
| Age (median) | 33.0 (IQR: 19-48) |
| Sex | |
| Female | 54 (28.49%) |
| Male | 133 (71.51%) |
| Occupation | |
| Iterant pupils (Almajiri) | 81 (43.33) |
| Formal school students | 12 (6.67) |
| Medical personnel | 12 (6.67) |
| Teachers | 6 (3.33) |
| Housemaids | 26 (13.21) |
| Others | 50 (26.6) |
| Mean duration from testing to admission (in days) | 3.1 (SD: 1.5) |
| Treatment | |
| Lopinavir-ritonavir | 64 (34.2%) |
| Chloroquine | 123 (65.8%) |
| Vitamin C | 187 (100%) |
| Azithromycin | 187 (100%) |
| Zinc | 187 (100%) |
| Median duration of hospitalization (in days) | 0 (IQR: 8-18) |
| Mode of exposure | |
| Contact with confirmed person | 167 (89.3%) |
| Travel to high risk area | 20 (10.7%) |
SD = Standard deviation; IQR=inter quartile range.
| Characteristic | symptomatic cases (47) | asymptomatic cases (140) | P-value |
|---|---|---|---|
| Age Years (SD) | 39.3 (2.2) | 31.2 (1.4) | 0.004# |
| Sex | |||
| Female | 15 | 39 | 0.72* |
| Occupation | 0.43* | ||
| Iterant pupils (Almajiri) | 0 | 81 | |
| Formal school students | 4 | 5 | |
| Medical personnel | 10 | 2 | |
| Teachers | 4 | 2 | |
| Housemaids | 11 | 17 | |
| Others | 18 | 34 | |
| Mode of exposure (Contact/travel) | 43/3 | 124/16 | 0.27* |
| Presence of any comorbidity/ Number without | 35/12 | 109/31 | 0.10* |
| (Mean/SD) duration of hospitalization | 13.38(SD: 5.38) | 12.27(SD: 5.84) | 0.24# |
SD: standard deviation; *Chi-square test; #T-test
Symptomatology
Of the 187 patients (study sample size) reviewed, 140 (74.8%) were asymptomatic. There was wide variability in symptom clusters comprising of fever, cough, sneezing, sore throat, runny nose, and body aches occurring in 80% of symptomatic patients (Table 3). Hypertension was the only additional comorbidity and was recorded in 26.7% of the study population. We noted significant differences in the frequency of occurrence of fever, cough, sneezing, sore throat, runny nose, and body aches between those discharged in less than ten days versus those discharged beyond ten days of hospitalization.
| Symptoms | Early viral clearance (Time to discharge from isolation center (<10 days) N=98 (52.4%) | Late viral clearance (Time to discharge from isolation center (≥10 days) n=89 (47.6%) | P-value |
|---|---|---|---|
| Fever (30)^ | 23 (23.5%) | 7 (7.9%) | 0.05 |
| Cough (26)^ | 12 (12.2%) | 14 (15.7%) | 0.15 |
| Sneezing (9)^ | 0 (0%) | 9 (10.1%) | 0.02 |
| Difficulty in breathing (10)^ | 3 (3.1%) | 7 (7.9%) | 0.12 |
| Sore throat (14)^ | 5 (5.1%) | 9 (10.1%) | 0.04 |
| Runny nose (8)^ | 3 (3.1%) | 5 (5.6%) | 0.04 |
| Body ache (10)^ | 6 (6.2%) | 4 (4.5%) | 0.08 |
| Fatigue (8)^ | 4 (4.1%) | 4 (4.5%) | 0.03 |
| Anosmia (11)^ | 5 (5.1%) | 6 (6.7%) | 0.09 |
| Agousia (1)^ | 0 (0%) | 1 (1.1%) | 0.09 |
| Vomiting (4)^ | 4 (4.1%) | 0 (0%) | 0.114 |
| Diarrhea (8)^ | 8 (8.2%) | 0 (0%) | 0.05 |
| Chest pain (6)^ | 2 (2.0%) | 4 (4.5%) | 0.05 |
| Loss of appetite (5)^ | 2 (2.0%) | 3 (3.4%) | 0147 |
| Presence of any co morbidity (37)^ | 21 (21.4) | 16 (17.9%) | 0.62 |
SARS-CoV-2 viral clearance was confirmed using real-time PCR; ^: Number of patients with itemized symptom or feature
Using Kaplan Meier estimation, we found no significant differences in time to discharge from the isolation center between younger (< 50 years) versus older patients (≥ 50 years) [10 days vs. 11 days respectively; p = 0.32] (Figure 2). We also did not observe a significant difference between those with comorbidity versus those without comorbidity (p = 0.26), (Figure 3) as well as between symptomatic versus asymptomatic patients (p = 0.43) (Figure 4). However, we noted a faster viral clearance rate in patients on lopinavir versus those on chloroquine [p = 0.048] (Figure 5). Using Cox Proportional Hazard model, we did not find age (HR: 0.99, 95% C.I:0.98 -1.01), sex (HR:0.95, 95% C.I:0.31-2.94) and source of infection (HR:1.54, 95% C.I: 0.86-2.73) to be predictors of time to discharge among the study cohort with COVID 19.

- Kaplan Meier estimation of time to discharge between those with age below and above age 50 years (with two-sided Log-rank test) Log-rank test p-value = 0.0818

- Kaplan Meier estimation of time to discharge between those with versus those without co-morbidity (with two-sided Log-rank test) Log-rank test p-value = 0.7814
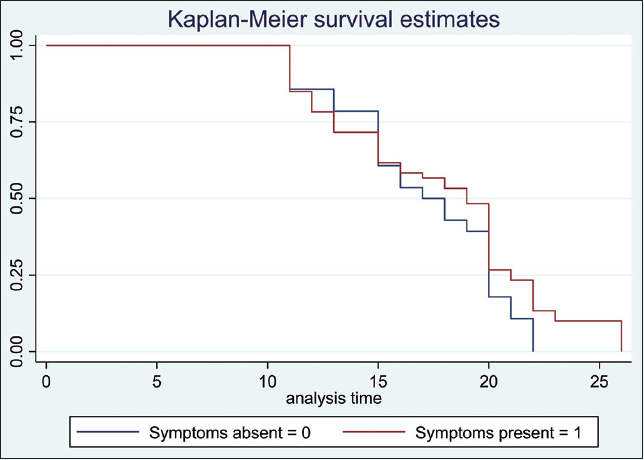
- Kaplan Meier estimation of time to discharge between those with and without symptoms (with two-sided Log-rank test) Log-rank test p-value = 0.1638
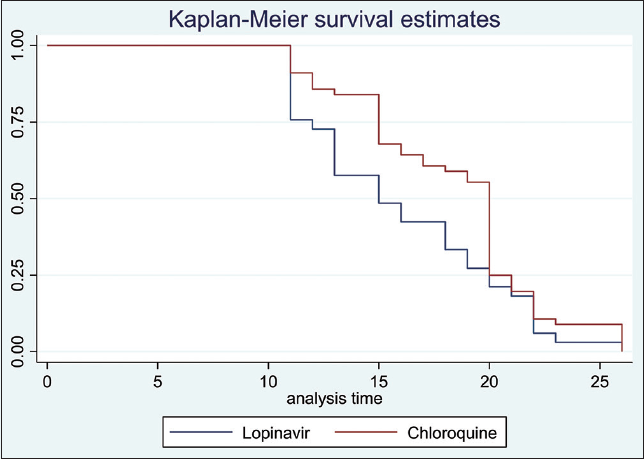
- Kaplan Meier estimation of time to discharge between those on Lopinavir versus those on chloroquine Log-rank test p-value = 0.048
Almajiri (itinerary students)
There were 81 Almajiri scholars among our patients (mean age = 14.8 years SD = 3.5 years). They were all males and asymptomatic, most of them had “late viral clearance” 46 (57.1%). There was no significant difference in duration of hospitalization at the isolation center between Almajiris versus Non- almajiris. (Log-rank p = 0.416) (Figure 6).
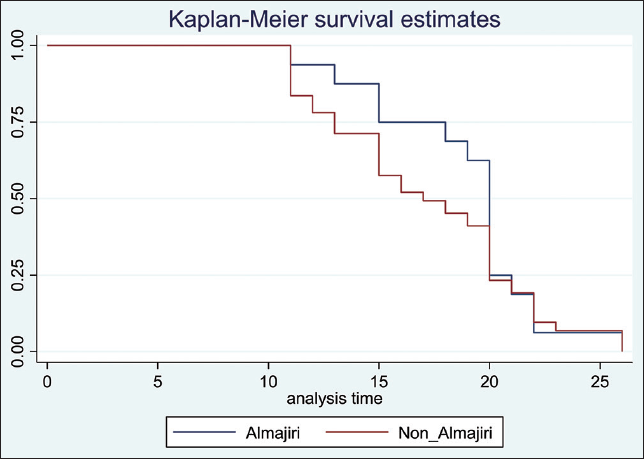
- Cumulative probabilities for time-to-discharge for COVID-19 positive Almajiris versus non-Almajiris displayed using Kaplan-Meier curves Log-rank test p-value = 0.416
Discussion and Implications for Translation
Whereas coronavirus has been known to cause respiratory infection, SARS-CoV-2 appears to be a novel virus that displays unique interactions with the immune system, which had hitherto been naïve to it. Consequently, profiling its clinical characteristics is vital to enhance early detection and curtail disease spread. To characterize the clinical phenotypes of COVID-19 patients in Kano, Northern Nigeria, this study retrospectively evaluated the clinical presentation among a cohort of non-severe hospitalized patients in Kano, Nigeria. We found the patients to be mostly young and largely presenting with fever, cough, difficulty in breathing, and sore throat, with most of them hospitalized for a little over a week. Earlier studies in Nigeria had similarly found young patients to be the most commonly affected.13,14The finding is similar to reports from Saudi Arabia among patients with non-severe disease.15,16 This is in contrast to the higher age reported among patients with mild-to-moderate clinical features in China.5,17The reason for the discrepancy might be largely related to the variance in admission criteria in the China study compared to ours, as well as differences in the age admixture of our cohort. Perhaps it might also reflect a wider gap in demographic age spread between these populations. It is not surprising to find a larger proportion of young persons in our cohort since our hospital a priori admitted persons with non-severe disease. On the contrary, severe disease is commoner among older patients.18
Vulnerabilities to contagions are especially related to the level and duration of exposure.19 The longer a person is exposed to a source of infection, the higher the likelihood of being infected. Traditional gender roles attributed to different gender strata in Africa demand men to work outdoors and secure resources for the family while women stay at home. Thus, men are more likely to be outdoors and run the risk of breaching social distancing requirements leading to a higher risk of SARS-CoV-2 infections among males.
The policy of our COVID-19 isolation center at the time of the study was to maintain in-patient care until a patient had negative RT-PCR viral test results. Therefore, the duration of hospitalization in our study of 10 (IQR 8-18) days represents a surrogate marker of time to RNA viral conversion. A study in Tunisia reported the median period to viral clearance to be 20 (IQR 17-32) days.20 Conversely, a study in Qingdao, China, reported 14 (IQR: 10-18) days as the median duration to viral conversion.20The differences across studies might be linked to the spectrum of disease severity typically observed in COVID-19 patients.
At our center, patient assignment to receive either chloroquine or Lopinavir was at the discretion of the physician on duty at the time of admission. We could, therefore, not objectively assess the results of the treatment impact observed in the study. Further, our results did not reveal any significant association between age, presence of comorbidity, and occurrence of symptoms with time to RNA conversion as outcome. It is important to contextualize the reasons behind our choices of drugs. There was no established evidence-based efficacious treatment for COVID 19 as at the time of care of the index cohort in 2020. All therapies then were exploratory but scientifically based on in-vitro drug performance, preclinical studies, and observational trends. Consequently, our choice of medications was anchored on prevailing anecdotal evidence similar to the practice in other countries.21-23Similar to several other studies, we found contact with an infected person to be the commonest means of SARS-CoV-2 spread.5,24-26 Respiratory infections are often transmitted by the propulsion of droplets of varying sizes. Social habits and the basic requirement of human interactions render human-to-human contact an inevitable mechanism for transmission. These dynamics have far-reaching implications for COVID-19 control measures, considering the need to enforce social distancing to mitigate further spread.
The itinerate Quranic school students, known locally as Almajiri children, are a vulnerable population. They are mostly displaced having left their place of birth, traveling far in search of knowledge, Kano had over 3,000 Almajiri schools, with approximately one million students.27 These students faced one of the highest medical and social challenges during the COVID-19 pandemic having no strong social support system. Furthermore, they were repatriated to their places of birth in spite of the difficulty in movement imposed by the then prevailing “lock-down.”28 Indeed, by virtue of the overcrowding in Almajiri schools and sustained displacement, the pupils were at a heightened risk of infection with SARS-CoV-2. Moreover, they serve as a veritable source of infection to the larger population.28
The aim of the study was to delineate COVID-19 phenotypes in Northern Nigeria based on a syndromic approach. Although the signs and symptom clusters observed in our patients are not specific to COVID-19, however, in the setting of an ongoing epidemic, they do offer an opportunity for guided clinical screening based on symptom clusters to enhance case detection efficiency.
Our study exhibits a number of limitations. First, only persons with non-severe SARS-CoV-2 infection were admitted to the MBSH isolation center, which limited the study sample size and ultimately power required to make firm conclusions. In addition, comparison with severe cases of COVID-19 disease phenotype was not feasible given the facility setting. Second, evaluation of co-morbidity status was mostly based on self-report, and hence, we could have missed some important comorbidities not volunteered by patients. Nonetheless, to the best of our knowledge, this study represents the largest that has described COVID-19 clinical phenotypes from Northern Nigeria.
In conclusion, the findings of this study have a bearing on the surveillance and diagnosis of COVID-19 in Nigeria. While the plethora of clinical features may not be limited to SARS-CoV-2 viral infection, the healthcare team should consider these symptom clusters in addition to cognate contact and travel history when confronted with a suspected case of COVID-19 infection.
Compliance with Ethical Standards
Conflicts of Interest:
The authors declare no competing interests.
Financial Disclosure:
Nothing to declare.
Ethical Approval:
Ethical approval was given from the ethics committee of Kano State Ministry of Health, Nigeria (Approval Number: (MOH/Off/797/T.I/2030).
Disclaimer:
None.
Acknowledgments:
None.
Funding/Support:
There was no funding for this study.
References
- A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727-733.
- [CrossRef] [PubMed] [Google Scholar]
- World Health Organization; (accessed )
- Nigeria Centre for Disease Control. (accessed )
- COVID-19 patients' clinical characteristics, discharge rate, and fatality rate of metaanalysis. J Med Virol. 2020;92(6):577-583.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical characteristics, predictors of symptomatic coronavirus disease 2019 and duration of hospitalisation in a cohort of 632 Patients in Lagos State, Nigeria. Niger Postgrad Med J. 2020;27(4):285-292.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical and demographic characteristics of COVID-19 patients in Lagos, Nigeria: a descriptive study. J Natl Med Assoc. 2021;113(3):301-306.
- [CrossRef] [PubMed] [Google Scholar]
- Descriptive epidemiology of coronavirus disease 2019 in Nigeria, 27 February-6 June 2020. Epidemiol Infect. 2020;148:e208.
- [CrossRef] [Google Scholar]
- National Centre for Disease Control. (accessed )
- Nigeria Centre for Disease Control. Published March 14, 2020 (accessed )
- National interim guideline for clinical management of COVID 19: version 2. Nigeria Centre for Disease Control. Published May 2, 2020 (accessed )
- [Google Scholar]
- Predictive ability of symptomatology in COVID-19 during active case search in Lagos State, Nigeria. Niger Postgrad Med J. 2020;27(4):280-284.
- [CrossRef] [PubMed] [Google Scholar]
- Outcomes of COVID-19 patients with comorbidities in southwest Nigeria. PLoS One. 2021;16(3):e0248281.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical, epidemiological, and laboratory characteristics of mild- to-moderate COVID-19 patients in Saudi Arabia: an observational cohort study. Eur J Med Res. 2020;25(1):61.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130(5):2620-2629.
- [CrossRef] [PubMed] [Google Scholar]
- Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239-1242.
- [CrossRef] [PubMed] [Google Scholar]
- Infectivity, susceptibility, and risk factors associated with SARS-CoV-2 transmission under intensive contact tracing in Hunan, China. Nat Commun. 202;12(1):2251.
- [CrossRef] [PubMed] [Google Scholar]
- Factors associated with a prolonged negative conversion of viral RNA in patients with COVID-19. Int J Infect Dis. 2021;105:463-469.
- [CrossRef] [PubMed] [Google Scholar]
- Factors associated with negative conversion of viral RNA in patients hospitalized with COVID-19. Sci Total Environ. 2020;728:138812.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061-1069.
- [CrossRef] [PubMed] [Google Scholar]
- Lopinavir-ritonavir in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2020;396(10259):1345-1352.
- [CrossRef] [PubMed] [Google Scholar]
- Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020;56(1):105949.
- [CrossRef] [PubMed] [Google Scholar]
- Community transmission of severe acute respiratory syndrome coronavirus 2, Shenzhen, China, 2020. Emerg. Infect Dis. 2020;26(6):1320-1323.
- [CrossRef] [PubMed] [Google Scholar]
- A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514-523.
- [CrossRef] [PubMed] [Google Scholar]
- Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382(13):1199-1207.
- [CrossRef] [Google Scholar]
- UNICEF Nigeria; (accessed )
- Public health implication of displacement of Almajiri children in specific states of Northern Nigeria amidst COVID-19 pandemic. Ethics Med Public Health. 2020;14:100544.
- [CrossRef] [PubMed] [Google Scholar]





