Translate this page into:
Predictors of Human Immunodeficiency Virus among Children 0–14 Years in Nigeria

*Corresponding author: Hamisu M. Salihu, Department of Epidemiology and Population Health, Kano Independent Research Centre Trust, Kano, Nigeria. E-mail: hamisu.salihu@kirct.com
-
Received: ,
Accepted: ,
How to cite this article: Abdullahi AA, Murtala HA, Aminu A, Abbas MA, Yusuf AL, Aliyu MH, et al. Predictors of HIV among children 0–14 years in Nigeria. Int J Transl Med Res Public Health. 2025;09:e009. doi: 10.25259/IJTMRPH_45_2025
Abstract
Background and Objective:
Children under the age of 15 represent 3% of the global population living with Human Immunodeficiency Virus (HIV), 9% of new HIV infections, and 12% of all deaths related to Acquired Immunodeficiency Syndrome (AIDS). This study describes the predictors of HIV among children aged 0–14 years in Nigeria.
Methods:
This cross-sectional study analyzed data from the 2018 Nigeria HIV/AIDS Indicator and Impact Survey, the largest household-based HIV survey globally, using a weighted sample of 84,130,443 children aged 0–14 years collected nationwide from July to December 2018. We employed descriptive statistics, Chi-square tests, and the Kruskal-Wallis test to assess differences in HIV prevalence between urban and rural pediatric populations, while logistic regression models were used to identify predictors of pediatric HIV, with results reported as adjusted odds ratios (AORs) and 95% confidence intervals (CIs).
Results:
Weighted data on a total of over 84 million children were analyzed, with a slightly higher median age and school enrollment observed among urban residents. HIV prevalence was low overall, at 0.1% in urban areas and 0.2% in rural areas. Among HIV-positive children, maternal awareness of HIV status was a key determinant: children whose mothers were aware of their HIV-positive status had a significantly lower likelihood of HIV infection (AOR: 0.02; CI: 0.01–0.06; p < 0.001). Conversely, having a living mother (AOR: 6.57; CI: 1.75–24.7; p = 0.005) and younger maternal age were were associated with an increased likelihood of pediatric HIV. Other sociodemographic and contextual factors, including the child’s age, sex, residence, school enrollment, and conflict zone status, were not significantly associated with pediatric HIV acquisition.
Conclusion and Implications for Translation:
The findings showed that maternal health significantly influences pediatric HIV outcomes. Children of HIV-positive mothers were less likely to acquire HIV, likely due to antiretroviral therapy and prevention of mother-to-child transmission programs. These findings emphasize the need for strengthened targeted interventions focusing on maternal care, HIV prevention, and healthcare access among vulnerable populations.
Keywords
Antiretroviral
Pediatric Human Immunodeficiency Virus
Viral Load Suppression
INTRODUCTION
Globally, pediatric Human Immunodeficiency Virus (HIV) remains a major public health challenge. As of 2023, an estimated 1.4 million (1.1–1.7 million) children aged 0–14 years were living with HIV, with approximately 22,000 (18,000–26,000) new infections and 15,000 (12,000–18,000) Acquired Immunodeficiency Syndrome (AIDS)-related deaths reported among this age group.[1] Despite progress in antiretroviral therapy (ART) scale-up, only 57% (41–75%) of HIV-positive children had access to treatment, compared to 77% (62–90%) of adults aged 15 and older.[1] This treatment disparity places HIV-infected children at greater risk of rapid disease progression, severe immunosuppression, and death if not promptly diagnosed and treated.
Nigeria bears the highest burden of pediatric HIV in West Africa,[2] 160,000 (140,000–180,000) children aged 0–14 years were living with HIV in 2023.[1] During the same year, 22,000 (18,000–26,000) new infections and 15,000 (12,000–18,000) AIDS-related deaths were recorded among children in this age group. Alarmingly, only 45,517 children received ART, reflecting a significant gap in treatment coverage and access to care.[1] HIV in children often manifests through recurrent respiratory infections, diarrhea, malnutrition, and septicemia, with presentations ranging from asymptomatic to advanced AIDS.[3,4] Early diagnosis and sustained ART are critical to reducing mortality and improving health outcomes in this vulnerable population.
To address the pediatric HIV epidemic, global and national strategies have been introduced to improve prevention, testing, and treatment. The UNAIDS 95-95-95 target, aimed at eliminating AIDS as a public health threat by 2030, seeks to ensure that 95% of people living with HIV know their status, 95% of those diagnosed receive sustained ART, and 95% of those on treatment achieve viral suppression.[5] Nigeria’s national HIV response aligns with this goal through a three-pronged strategy combining biomedical, behavioral, and structural prevention services.[6] This integrated approach is also consistent with the Global HIV Prevention Coalition’s roadmap, which aims to provide at least 90% of the population, including key and vulnerable groups, with access to comprehensive HIV prevention services by 2030.[7] Another critical component of Nigeria’s strategy is to achieve 90% ART coverage among HIV-positive women by 2025, which is pivotal for preventing mother-to-child transmission (PMTCT) during pregnancy and childbirth.[8]
In Nigeria, the country with the second-largest HIV epidemic in the world,[6] pediatric HIV poses a persistent challenge to public health. While several studies have characterized the clinical course and treatment outcomes of pediatric HIV, less is known about the socio-demographic and maternal factors associated with HIV infection among children.[7] A study conducted among young people in Tanzania identified education level, marital status, and mobility as significant factors associated with HIV infection in adults.[9] Similarly, research from India found that among women, being widowed was the strongest risk factor for HIV, while among men, being between the ages of 25 and 45 and having a low level of education were linked to a higher risk of infection.[10] Existing literature primarily focuses on adult HIV acquisition risk parameters often neglecting broader pediatric HIV risk determinants such as maternal age, HIV awareness, and social factors like parental survival. Furthermore, disparities in healthcare access between urban and rural populations remain poorly understood, particularly as they relate to pediatric HIV outcomes. There is a clear need to explore these contextual and structural drivers to inform more effective and targeted interventions.
Pediatric HIV remains a persistent public health challenge in Nigeria, with mother-to-child transmission accounting for most new infections despite global progress toward UNAIDS targets. Critical gaps in PMTCT coverage, particularly in maternal HIV awareness and rural-urban service access, continue to drive transmission, yet few studies have examined the role of maternal survival status or geographic disparities in this context. To address this gap, we conducted a retrospective analysis of Nigeria HIV/AIDS Indicator and Impact Survey (NAIIS) data from children aged 0–14 years, testing how maternal characteristics (HIV status, age, and survival) interact with child-level and contextual factors to influence pediatric HIV outcomes. By focusing on these understudied predictors, our study provides novel evidence to guide targeted PMTCT interventions and accelerate progress toward eliminating childhood HIV in Nigeria.
METHODS
Study Design
This is a cross-sectional study based on data from the NAIIS, conducted nationwide from July to December 2018. The NAIIS was a population-based, nationally representative survey designed to provide accurate estimates of HIV prevalence and viral load suppression, as well as the burden of related co-infections including hepatitis B, hepatitis C, and tuberculosis. Comprehensive details of the survey methodology are available in the NAIIS Final Report.[11] The NAIIS is the largest nationwide HIV survey of its era.
This study is reported in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology statement, an international guideline designed to improve the transparency and quality of reporting in observational studies.[12]
Study Population and Sampling
This analysis focused on children aged 0–14 years drawn from the NAIIS dataset. The survey excluded institutional populations such as those residing in military barracks, correctional facilities, and boarding institutions. The sampling framework was derived from the 2006 Nigerian National Population and Housing Census, comprising 662,855 Enumeration Areas (EAs). A two-stage, stratified cluster sampling approach was employed: first, EAs were selected with probability proportional to size, and second, households within each EA were systematically sampled. All eligible household members, including children and their guardians, were invited to participate in structured interviews and biological sample collection for HIV testing.
Study Variables
Outcome variable
The primary outcome was the HIV status of the child, determined through laboratory-confirmed testing using dried blood spot specimens and classified as HIV-positive or HIV-negative according to the national testing algorithm.
Explanatory variables
Sociodemographic variables included the child’s age, sex, school enrollment status, place of residence (urban/rural), region (North/South), household wealth quintile, and conflict zone classification Maternal variables included the mother’s age, HIV status, and survival status. Clinical variables considered whether the child had ever had a CD4 test, were currently receiving ART, or was on prophylaxis with co-trimoxazole (Septrin). Conflict status was defined in previous research, which categorized states such as Benue, Borno, Plateau, Yobe, and Zamfara as conflict-affected states.[13]
Data Collection and Management
Data collection was conducted by 1,935 trained field personnel including interviewers, counselors, laboratorians, and supervisors. Electronic questionnaires were administered during household visits using tablets programmed with CSPro software. Biomarker and survey data were synchronized via Bluetooth and uploaded daily to secure servers using FTPS protocols. Laboratory data were processed using the Laboratory Data Management System, with comprehensive quality control procedures in place. Secure backup protocols included encrypted storage on external devices.
Data Analysis
All statistical analyses were conducted using R software (version 4.4.3). Survey weights were applied to account for the complex sampling structure, including stratification, clustering, and differential non-response. The survey package in R was used for variance estimation and inference. Descriptive statistics were utilized to compare socio-demographic characteristics of urban and rural children, with further sub-analysis for maternal and child related characteristics of HIV-positive children. Categorical variables were compared using Pearson’s Chi-square test, while continuous variables were analyzed using Wilcoxon rank-sum tests. To identify factors associated with HIV infection among children, survey-weighted multivariable logistic regression models were fitted. Adjusted odds ratios (AORs) with 95% confidence intervals (CIs) were reported.
RESULTS
We analyzed weighted samples of 84,130,443 children comprising 37,338,667 (44%) urban and 46,791,776 (56%) rural residents [Table 1]. The median age was slightly higher among urban children (8 years, interquartile range [IQR]: 4–11) compared to their rural counterparts (7 years, IQR: 4–11, p < 0.001). The gender distribution was similar across both groups, with males comprising 51% and 52% in urban and rural settings, respectively (p < 0.001). School enrollment was significantly higher among urban (72%) than rural children (56%) (p < 0.001). A larger proportion of rural children resided in conflict zones (12%) compared to urban children (8.5%) (p < 0.001), and children in rural areas were predominantly from the northern region (68%) versus 53% of urban children (p < 0.001). Wealth distribution was markedly skewed, with rural children more likely to fall within the lowest (34%) and second (29%) wealth quintiles compared to only 7.4% and 12% of urban children, respectively (p < 0.001). HIV prevalence among children was lower (0.1% vs. 0.2), although the difference was not statistically significant positive (p = 0.82).
| Characteristic | Urban (n=37,338,667) | Rural (n=46,791,776) | P-value |
|---|---|---|---|
| Age (years), Media n(IQR) | 8 (4–11) | 7 (4–11) | <0.0011 |
| Gender, n(%) | <0.0012 | ||
| Male | 18,911,240 (51) | 24,342,123 (52) | |
| Female | 18,427,427 (49) | 22,449,653 (48) | |
| Child enrolled in school, n(%) | <0.0012 | ||
| Yes | 26,993,448 (72) | 26,140,600 (56) | |
| No | 10,345,219 (28) | 20,651,176 (44) | |
| Conflict vs. conflict status, n(%) | <0.0012 | ||
| Non-conflict zone | 34,149,734 (91) | 41,331,185 (88) | |
| Conflict zone | 3,188,933 (8.5) | 5,460,591 (12) | |
| Geopolitical zone, n(%) | <0.0012 | ||
| North | 19,727,869 (53) | 31,828,749 (68) | |
| South | 17,610,798 (47) | 14,963,027 (32) | |
| Wealth quintile, n(%) | <0.0012 | ||
| Lowest | 2,779,377 (7.4) | 15,807,731 (34) | |
| Second | 4,316,650 (12) | 13,627,828 (29) | |
| Middle | 7,106,245 (19) | 9,623,859 (21) | |
| Fourth | 10,320,409 (28 | 5,683,010 (12) | |
| Highest | 12,815,986 (34) | 2,049,347 (4.4) | |
| Child HIV status, n(%) | 0.82 | ||
| HIV positive | 15,002 (0.1) | 17,374 (0.2) | |
| HIV negative | 10,663,526 (100) | 11,370,873 (100) | |
| Missing | 26,660,139 | 35,403,529 |
Among HIV-positive children (n = 32,376, [Table 2]), the median maternal age and children’s age groups were similar between urban and rural residents. Additionally, a greater proportion of HIV-positive children in rural areas had HIV-positive mothers (57%) compared to those in urban areas (40%) (p = 0.4). A higher proportion of rural children had both parents alive, with 88% having living fathers and 90% having living mothers, compared to 68% and 77% in urban areas, respectively. Notably, rural children were significantly more likely to have undergone CD4 count testing (74% vs. 0%, p = 0.033), be on Septrin prophylaxis (83% vs. 12%, p = 0.033), and currently receiving ART (100% vs. 12%, p = 0.003) than urban children [Figure 1].
| Characteristic | Urban (n=15,002) | Rural (n=17,374) | P-value |
|---|---|---|---|
| Mother age, Media n(IQR) | 35 (29–41) | 35 (26–38) | 0.51 |
| Missing | 2,904 | 3,892 | |
| Mother's awareness of HIV status, n(%) | 0.42 | ||
| HIV Positive | 4,065 (40) | 7,350 (57) | |
| HIV Negative | 6,036 (60) | 5,542 (43) | |
| Missing | 4,901 | 4,483 | |
| Father alive, n(%) | 0.22 | ||
| Yes | 10,213 (68) | 15,293 (88) | |
| No | 4,789 (32) | 2,081 (12) | |
| Mother alive, n(%) | 0.32 | ||
| Yes | 11,624 (77) | 15,629 (90) | |
| No | 3,378 (23) | 1,745 (10) | |
| Childs ever had CD4 test, n(%) | 0.0332 | ||
| Yes | 0 (0) | 1,796 (74) | |
| No | 2,717 (100) | 627 (26) | |
| Missing | 12,285 | 14,951 | |
| Child on septrin, n(%) | 0.0332 | ||
| Yes | 338 (12) | 2,012 (83) | |
| No | 2,379 (88) | 411 (17) | |
| Missing | 12,285 | 14,951 | |
| Child currently on ART, n(%) | 0.0032 | ||
| Yes | 338 (12) | 2,423 (100) | |
| No | 2,379 (88) | 0 (0) | |
| Missing | 12,285 | 14,951 |
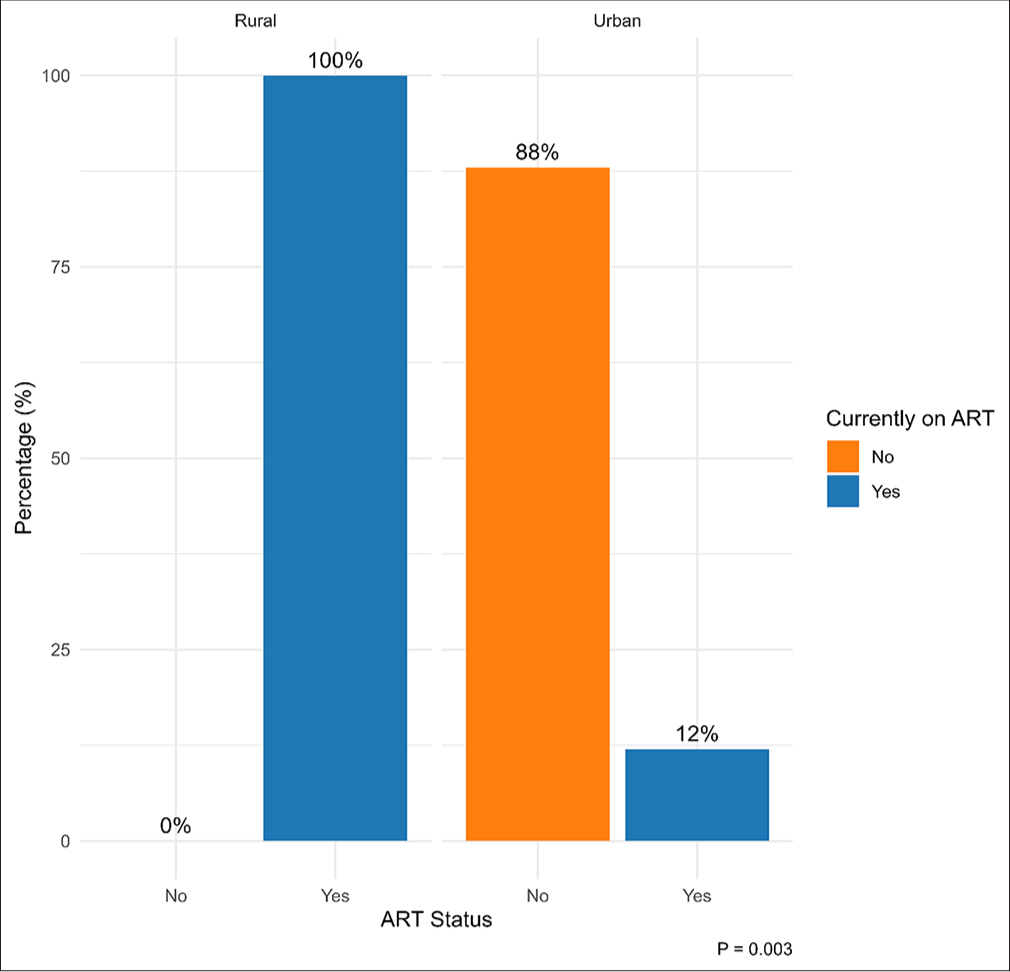
- Proportion of HIV-positive children currently on ART by place of residence. HIV: Human immunodeficiency virus, ART: Antiretroviral therapy.
Multivariable logistic regression revealed that children whose mothers were aware of their HIV-positive status were 98% less likely to have HIV infection compared to those whose mothers were HIV-negative (AOR: 0.02, CI: 0.01–0.06, p < 0.001). Having a living mother was also significantly associated with a more than sixfold higher likelihood of HIV infection (AOR: 6.57, CI: 1.75–24.7, p = 0.005). Conversely, for every year increase in maternal age, the likelihood of child HIV infection was reduced by 5% (AOR: 0.95, CI: 0.92–1.00, p = 0.031). Other factors, including the child’s age, gender, school enrollment, urban residence, geopolitical zone, father’s survival status, and conflict zone residence were not found to be significantly associated with HIV infection among children aged 0–14 years.
DISCUSSION
In a weighted cohort of 84,130,443 children aged 0–14 years, we found that maternal factors played a major role in determining children’s HIV status. These factors included maternal awareness of HIV status, age, and mother’s survival [Table 3]. Contrary to the common expectations, our study showed that children with HIV-positive mothers were significantly less likely to be HIV-positive. This is in contrast to the widely cited report suggesting that, without medical intervention, 15–45% of pregnant women living with HIV will transmit the virus to their children.[14] Previous studies also associated maternal HIV infection with infant’s increase susceptibility to HIV infection in early life.[15] Additionally, it is well established in the literature that perinatally infected infants usually experience rapid disease progression within the 1st months of life. This understanding would typically lead to an expectation of higher HIV prevalence among younger children (0–4 years).
| Characteristic | AOR | 95% CI | P-value |
|---|---|---|---|
| Childs age | 1.05 | 0.95, 1.16 | 0.3 |
| Mothers age | 0.95 | 0.92, 1.00 | 0.031 |
| Gender | |||
| Male | — | — | |
| Female | 1.03 | 0.44, 2.45 | >0.9 |
| Child enrolled in school | |||
| Yes | — | — | |
| No | 0.76 | 0.34, 1.74 | 0.5 |
| Urban status | |||
| Urban | — | — | |
| Rural | 0.91 | 0.39, 2.14 | 0.8 |
| Country zone | |||
| North | — | — | |
| South | 0.54 | 0.16, 1.80 | 0.3 |
| Mothers HIV status | |||
| HIV Negative | — | — | |
| HIV Positive | 0.02 | 0.01, 0.06 | <0.001 |
| Father alive | |||
| No | — | — | |
| Yes | 1.90 | 0.55, 6.51 | 0.3 |
| Mother alive | |||
| No | — | — | |
| Yes | 6.57 | 1.75, 24.7 | 0.005 |
| Conflict status | |||
| Non-conflict zone | — | — | |
| Conflict zone | 0.31 | 0.09, 1.06 | 0.063 |
AOR: Adjusted odds ratio, CI: Confidence interval, HIV: Human immunodeficiency virus.
However, our study found a higher prevalence of HIV among older children (10–14 years age group). This pattern suggests that mother-to-child transmission is an unlikely primary source of infection among these children. Rather it might likely be due to horizontal transmission through unsafe medical and cultural practices, such as non-sterile traditional piercing and scarification, a transmission route previously documented in pediatric HIV epidemics in settings like Pakistan.[16] Furthermore, the findings may also reflect improvements in the prevention of mother-to-child transmission programs, particularly among mothers who are aware of their HIV status and actively receiving HIV care, thereby reducing vertical transmission.
Additionally, our analysis revealed that having a living mother was significantly associated with an increased likelihood of pediatric HIV infection in children, whereas increasing maternal age was inversely associated with the risk of pediatric HIV. These highlight the complex and potentially counterintuitive pathways through which maternal health dynamics influence both vertical and horizontal HIV transmission.
Several other secondary findings require further discussion. Notably, HIV-positive children residing in rural areas reported significantly greater access to HIV-related services, such as CD4 count testing, Septrin prophylaxis, and ART initiation compared to residents of urban areas. This trend aligns with earlier reports from previous studies, which showed that individuals receiving HIV care in rural settings were more likely to be managed by providers with less caseloads, potentially allowing for more personalized care and improved service delivery.[17] Also, a similar Nigerian study had reported that people living with HIV, residents of rural areas, were more likely to receive an early HIV diagnosis compared to those in urban areas.[18] This might be explained by the fact that community-based outreach programs and active case finding often prioritize rural areas, thus resulting in earlier identification and prompt commencement of treatment.
Another unexpected finding was the higher prevalence of pediatric HIV in rural areas compared to urban areas (0.2% vs. 0.1%). This observation mirrors recent studies that found a higher burden of HIV among adult women living in rural areas of Nigeria.[18] The elevated rural prevalence could be linked to lower levels of education, widespread poverty, and the persistence of harmful traditional practices, such as tribal markings, body piercings, and uvulectomy, which are common in rural communities and may increase the risk of HIV transmission. Supporting this, our study found that only 56% of children residents of rural areas were enrolled in school, compared to 72% in urban areas. Additionally, a substantial proportion of rural children (34%) belonged to households in the lowest wealth quintile compared to 7.4% of urban areas, demonstrating the extensive socio-economic disadvantages in rural areas. While our analysis found higher PMTCT coverage in some rural areas, this likely reflects targeted donor-supported initiatives rather than superior systemic healthcare infrastructure. Urban-rural disparities in Nigeria remain complex and context-specific, with substantial variation across states and healthcare facilities.
Limitations
An important limitation of this study is its cross-sectional design, which restricted our ability to infer causality between identified predictors and HIV status. Additionally, some relevant covariates, such as breastfeeding history, mode of delivery, maternal CD4, and viral load, were not captured. This might have had an impact on the robustness of the adjusted odd ratios, potentially omitting key factors that influence HIV transmission in children.
CONCLUSION AND IMPLICATIONS FOR TRANSLATION
This study highlights the complex and potentially counterintuitive pathways through which maternal health dynamics influence both vertical and horizontal HIV transmission. It also emphasizes the need for context-specific public health strategies that address child HIV and maternal health through preventive education and safe cultural and healthcare practices to curb the pediatric HIV burden.
Key Messages
1) Pediatric HIV in Nigeria remains a critical public health challenge, driven largely by mother-to-child transmission (MTCT) and gaps in prevention programs. 2) This study highlights the understudied role of maternal survival status, age, and geographic disparities in shaping pediatric HIV outcomes, using data from the Nigeria HIV/AIDS Indicator and Impact Survey (NAIIS). 3) Our findings call for targeted interventions that address maternal HIV awareness, strengthen PMTCT (prevention of MTCT) services in rural communities, and integrate support for orphaned children. By focusing on these contextual and structural drivers, this study provides actionable evidence to accelerate progress toward eliminating pediatric HIV in Nigeria.
Acknowledgments
None.
COMPLIANCE WITH ETHICAL STANDARDS
Conflicts of Interest: Dr. Salihu and Ms. Dongarwar are members of the editorial board of the journal. Financial Disclosure: Nothing to declare. Funding/Support: The study received funding from the President’s Emergency Plan for AIDS Relief (PEPFAR), the US Centers for Disease Control and Prevention, and the Global Fund for AIDS, TB, and Malaria. Ethics Approval: Ethical approval was granted by the US Centers for Disease Control and Prevention (CDC; protocol #7103), the University of Maryland, Baltimore Institutional Review Board, and the Nigerian National Health Research Ethics Committee, dated NAIIS 2018. Declaration of Patient Consent: The authors certify that they have obtained all appropriate patient consent. Use of Artificial Intelligence (AI)-Assisted Technology for Manuscript Preparation: The authors confirm that there was no use of Artificial Intelligence (AI)-Assisted Technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI. Disclaimer: None.
References
- Nigeria - UNAIDS. 2025. [cited 2025 May 11]. Available from: https://www.unaids.org/en/regionscountries/countries/nigeria
- [Google Scholar]
- A child was infected with HIV every two minutes in 2020 – UNICEF. [cited 2025 May 11]. Available from: https://www.unicef.org/nigeria/press-releases/child-was-infected-hiv-every-two-minutes-2020-unicef
- [Google Scholar]
- Pediatric HIV in Kano, Nigeria. Niger J Clin Pract. 2013;16(4):521.
- [CrossRef] [PubMed] [Google Scholar]
- The clinical features of paediatric HIV/AIDS at presentation at the University of Abuja Teaching Hospital, Gwagwalada. Niger J Med. 2008;17(4):433-8.
- [CrossRef] [PubMed] [Google Scholar]
- Frontiers - Challenges in reaching the UNAIDS 95-95-95 targets in Sub-Saharan Africa: Status, innovations, and pathways forward. [cited 2025 May 11]. Available from: https://www.frontiersin.org/research-topics/66743/challenges-in-reaching-the-unaids-95-95-95-targets-insub-saharan-africa-status-innovations-and-pathways-forward
- [Google Scholar]
- HIV prevention cascade theory and its relation to social dimensions of health: A case for Nigeria. HIV AIDS (Auckl). 2019;11:193-200.
- [CrossRef] [PubMed] [Google Scholar]
- Maternal socio-demographic factors and mother-to-child transmission of HIV in the North Region of Cameroon. Int J MCH AIDS. 2023;12:e593.
- [CrossRef] [PubMed] [Google Scholar]
- Achieving UNAIDS 90-90-90 targets for pregnant and postpartum women in sub-Saharan Africa: Progress, gaps and research needs. J Virus Erad. 2018;4(Suppl 2):33-9.
- [CrossRef] [PubMed] [Google Scholar]
- HIV Infection among Young People in Northwest Tanzania: The role of biological, behavioural and socio-demographic risk factors. PLoS One. 2013;8(6):e66287.
- [CrossRef] [PubMed] [Google Scholar]
- Socio-demographic risk factors associated with HIV infection in patients seeking medical advice in a rural hospital of India 2012. J Public Health Res. 2012;1:79-82.
- [CrossRef] [PubMed] [Google Scholar]
- The burden of HIV, Hepatitis B and Hepatitis C by armed conflict setting: The Nigeria AIDS indicator and impact survey, 2018. Ann Glob Health. 2021;87(1):53.
- [CrossRef] [PubMed] [Google Scholar]
- Global HIV programme. Mother-to-child transmission of HIV. [cited 2025 May 10]. Available from: https://www.who.int/teams/global-hiv-hepatitis-and-stis-programmes/hiv/prevention/mother-to-child-transmission-of-hiv
- [Google Scholar]
- Effect of Maternal HIV infection on infant development and outcomes. Front Virol. 2022;2:885246.
- [CrossRef] [Google Scholar]
- A pediatric HIV outbreak in Pakistan. East Mediterr Health. 2024;30(1):60-7.
- [CrossRef] [PubMed] [Google Scholar]
- The Care of HIV-Infected adults in rural areas of the United States. JAIDS J Acquir Immune Defic Syndr. 2001;28(4):385.
- [CrossRef] [PubMed] [Google Scholar]
- On characterizing gender and locational composition of adult PLHIV in Nigeria: Implications for HIV programming. PLoS Glob Public Health. 2024;4(8):e0002863.
- [CrossRef] [PubMed] [Google Scholar]





