Translate this page into:
Birth-Weight and Risk of Breast Cancer: A Systematic Review Study
✉Corresponding author email: aosanjorin@gmail.com
Abstract
Introduction:
Observational studies have linked the risk of breast cancer to birth-weight; however, the findings are not consistent. Therefore, the objective of this study was to investigate and quantify the level of risk of breast cancer associated with birth-weight among women.
Methods:
A systematic literature search was conducted from 1990-2016 using the following databases: PUBMED, DH-Data, EMBASE, MEDLINE, PSYCINFO and GOOGLE SCHOLAR. A total of 14 relevant articles were identified through the systematic review, out of which 5 were suitable for meta-analysis. The computer software Review Manager (RevMan) 5.2 was used for the meta-analysis.
Results:
Most of the studies reviewed reported significant increased risk of breast cancer among participants with high birth-weight. There were indications that this relationship was more pronounced among premenopausal women. In addition, the meta-analysis further revealed that women with sub-optimal birth-weight (<3,500 g) are at lesser risk of developing breast cancer when compared with optimal birth- weight (3,500-4,500 g) OR= 1.17 (95% CI 0.98, 1.39); while optimal birth-weight (3,500-4,500 g) women are at lesser risk of developing breast cancer when compared to women with above-optimal birth-weight (>4,500 g) OR=0.87 (95% CI 0.66, 1.15).
Conclusion and Implications for Translation:
This study revealed that the risk of breast cancer increases with increasing high birth weight, especially among premenopausal women, thus suggesting early onset of breast cancer in this group. There was a clear relationship between high birth-weight and risk of breast cancer; the developmental origin of health and diseases theory as postulated by Baker may be the strongest biological mechanism to explain this finding. Prevention programs through health education and early diagnosis strategies targeted at this group might be promising strategies to tackle the global burden of breast cancer.
Keywords
Breast Cancer
Birth-Weight
Level of Risk
Developmental Origin of Disease
Systematic Review
Introduction
Breast cancer, a non-communicable disease, is the most common cancer in women and the most prevalent cause of cancer death among women globally.1,2,3 Also, it is the second cause of cancer death in more developed countries (198,000 deaths, 15.4% of total), while it is the most frequent cause of death in women in less developed countries (324,000 deaths, 14.3% of total).4 The exact etiology of breast cancer is largely unknown; however, series of studies have linked factors such as age, gender, family history, early menarche, late menopause, oral contraceptives, alcohol consumption, obesity in postmenopausal women, with increased risk of breast cancer.5,6,7
Meanwhile, there has been increasing evidence about the impact of in utero development on the risk of developing diseases in later life. Studies have shown that birth-weight, a surrogate indicator of in utero development, is closely associated with the risk of developing diseases such as diabetes, cardiovascular diseases, stroke, cancer, infectious diseases, and minor illnesses.8,9,10,11,12 In 1995, Barker postulated the theory of developmental origin of diseases as the underpinning biological mechanism for these relationships.13,14,15 Recently, there has been an emerging concept that the increased risk of diseases associated with birth- weight in infants, children, and adults is in a J-shaped pattern. Therefore, the risk of diseases associated with birth-weight is argued to increase when birth-weight is less than 3,500 g, decrease when birth-weight is between 3,500-4,500 g, and increase again when birth-weight is higher than 4,500 g. In relation to breast cancer, research has shown that both low and high birth-weight are also associated with increased risk of developing breast cancer in later life.16,17,18,19,20,21 Generally, this concept is under-researched and there has been conflicting findings from the few studies that have explored this in the literature.22,23,24,25
Therefore, the aim of this study was to investigate and provide robust evidence on the possibility of a J-Shaped pattern in the risk of breast cancer in association with birth-weight, by appraising relevant studies through a systematic review using the meta-analytical method.
Methods
This study was carried out using the guideline for conducting and reporting Meta-analysis of Observational Studies in Epidemiology (MOOSE).26
Literature Search
A thorough systematic literature search was conducted, spanning studies from 1990 to 2016 using the following search terms: ‘Birth-Weight’, ‘Low Birth-Weight’, ‘High Birth-Weight’, ‘Breast Malignancy’, ‘Breast Cancer’, ‘Risk Factors’, ‘Level of Risk’ and ‘Association’, ‘Relationship between Breast Cancer’, ‘Birth-Weight’ ‘Case-control’ ‘Cohort’ to identify relevant studies. The following databases; PUBMED, DH-DATA, EMBASE, MEDLINE, PSYCINFO, and GOOGLE SCHOLAR, were used to search for original articles that assessed the relationship between birth-weight and breast cancer. A snowballing approach was used to identify relevant studies. The studies generated while searching the databases unavoidably consisted of duplication and irrelevant studies, hence, the advanced search options of the databases were used to narrow the search to more relevant studies (Figure 1).
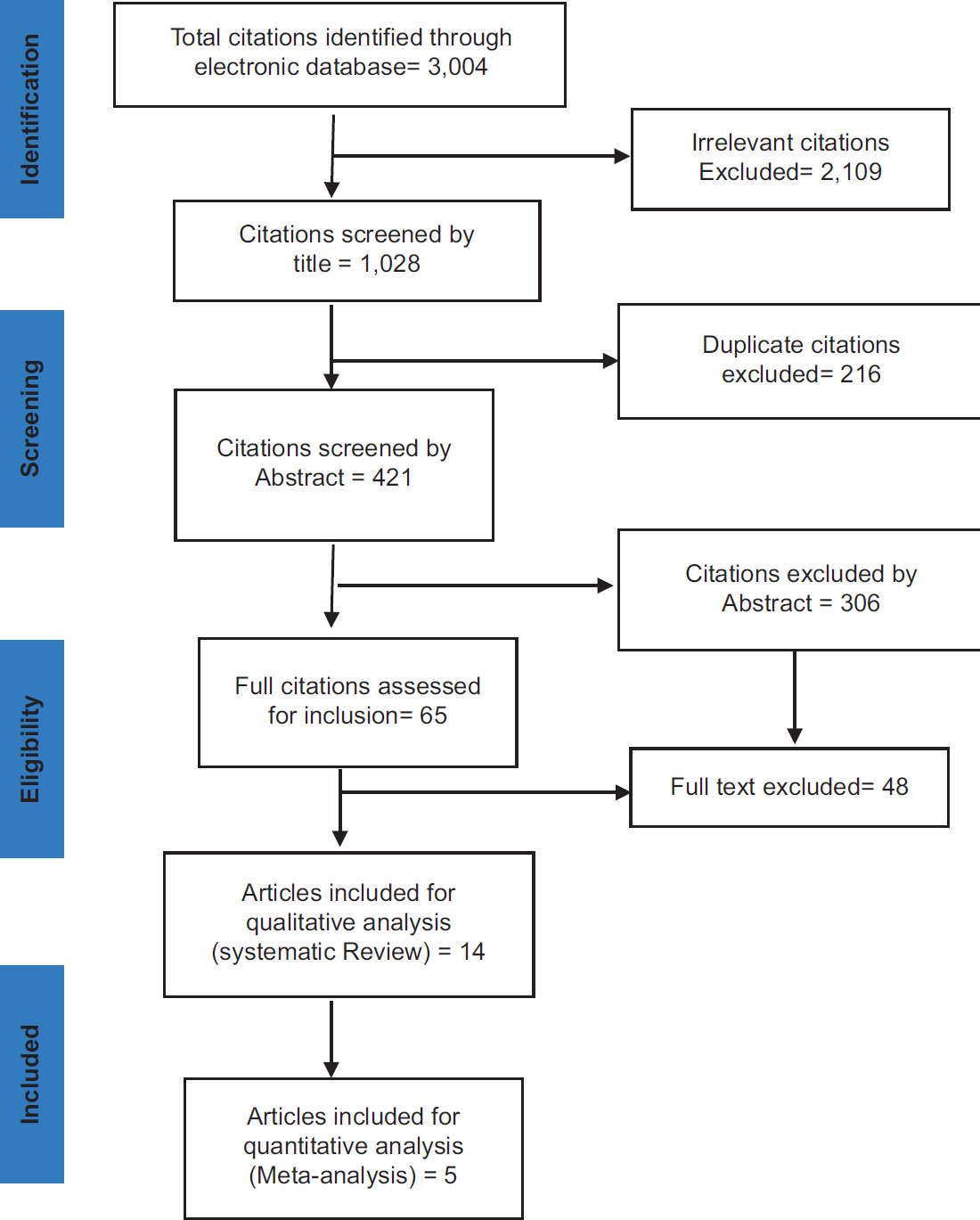
- Flow Diagram of Study Selection According to Prisma Flow Diagram
Studies Selection
The following inclusion criteria, defined a priori, were used to determine inclusion or exclusion of studies into the systematic review and meta-analysis: original article that have assessed the relationship between birth-weight and risk of breast cancer, observational (cohort or case-control) studies, published and/or grey literature between 1990-2016, studies that reported sufficient statistical information to permit estimation of appropriate effect size, and that the measured outcome must have been diagnosis of breast cancer in women. In order to ensure methodological appraisal, included studies must have documented the diagnosis of breast cancer using a standard diagnostic tool. Further quality appraisal was conducted using STROBE Checklist for Cohort, Case-Control and Observational Studies.27 All the 14 included studies were assessed across the 22 criteria of THE STROBE statement guideline. Each study was awarded 0 or 1 if the criterion was not met or if the criterion was met respectively; therefore, the highest obtainable score was 22. A study was graded as low if the score was <12, medium if the score was between 12-18, and high quality if the score was >18. The outcome of the quality appraisal was not used to determine the inclusion criterion, in order to capture all the studies that have assessed desired outcomes. However, it was used to interpret findings in this study.
Data Extraction and Analysis
All the relevant data involved in the systematic review and meta-analysis was extracted using a standardized form by the Principal Investigator (PI) (OS). The meta-analytical process involved two groups of dichotomous comparison. The total number of subjects with suboptimal birth-weight (>3,500 g), optimal birth- weight (3,500-4,500 g), and above optimal (>4,500 g), was extracted from all the included studies. Further, data on the numbers of participants with morbidity or mortality due to breast cancer were also extracted according to the birth-weight group stated above. This process was repeated twice by the PI and crossed checked by the SA using the same standardised form; the areas of differences were settled. (Supplement 1 for details of information and data extracted). In addition, the authors of the articles were contacted via email for clarifications when necessary.
In the qualitative analysis, the data extracted from the included studies were organized by emerging themes, and manually analyzed using a narrative synthetic approach. The Mantel-Haenszel method was used to estimate the mean effect estimates across the included studies, due to its robustness and higher precision, in combination of weighted average.28 The random effect model was used due to the expected variations among the included studies, while the odd ratio was used to interpret and measure the risk estimate across studies. A sub-group analysis was done to access the variability in the included study design. The Chi-Square (X2), p-value, I2, and the odds ratio (OR) were the statistical tests used in this study. Hence, X2 and I2 was used to assess the heterogeneity between studies. A funnel plot was generated from the forest plot, which was then used to assess publication bias. The Review Manager (RevMan) 5.2 from Cochrane Library was used to perform the quantitative analysis (meta-analysis).29
Results
The searches generated 3,004 articles. These were then screened by their titles, yielding the exclusion of 2,109 titles that were determined as irrelevant, and of 216 titles that were deemed as duplicates. In total, 421 articles were screened by their abstracts, 306 of which were excluded. Therefore, a total of 65 full-text relevant articles were retrieved for detailed reading and assessment with the eligibility criteria. Following review, 48 articles did not meet at least one of the selection criteria, while data could not be extracted from 3 articles due to incomplete presentation of birth-weight range. Hence, 14 studies were eligible and included in the systematic review study, of which only 5 studies were eligible and included in the meta-analysis.
Study Characteristics
The following information was retrieved from the included articles: Authors' name, title of the article, publication year, country, study design, measured exposure and outcomes, source of data, follow-up period, study size, major findings and co-founding factors (Table 1). All the included studies were recent studies; the oldest year of publication within the selected studies was 2000. The studies were all conducted within the United States, United Kingdom and other European countries (Table 1).
| Study (Author, Year of Publication, Country) | Study Title / Design | Follow-up Period | No. of Breast Cancer Cases | Study Population | Source of Birth- Weight Information | Results / Findings | Adjustment for other Covariates |
|---|---|---|---|---|---|---|---|
| Troisi et al., 2006 UK | Birth weight and breast cancer risk / Cohort study | 1992-2001 | 97 | 4,505 | The NCI DES Combined Cohort Study National Death Index (NDI)-Plus | There was no association between birth weight and breast cancer risk comparing women who weighed 3,000 g (rate ratio (RR)¼0.93) or 4,500 g (RR¼1.09), with women who weighed 3,000-3,499 g at birth (P for trend¼0.69). There was no obvious pattern in the association of gestational age with breast cancer incidence (P for trend¼0.66). | Mother's age, and gestational age |
| Ahlgren et al., 2004 Demark | Birth weight and risk of cancer / Cohort study | 1936-1975 | 12,540 | 106,504 | Danish Cancer Registry | Breast cancers demonstrated a positive linear association with birth weights. | None |
| Kaijer et al., 2003 Sweden | Preterm birth, birth weight, and subsequent risk of female breast cancer / Cohort study | 1925-1949 | 1,483 | Swedish Cancer Register | The overall risk of cancer among the women was not increased. The risk of breast cancer was neither associated with preterm birth nor with low birth weight, but a birth weight of more than 3,000 g was associated with an increased risk of breast cancer | Gestational age | |
| Mæhle et al., 2010 Norway | Birth length and weight as predictors of breast cancer prognosis / Cohort study | 1910-2003 | 331 | 331 | Norwegian Cancer Registry, The Central Person Registry | The study focused on the association of birth length and breast cancer risk (hazard ratio 1.92, 95% confidence interval, 1.09-3.41) of dying from breast cancer compared to patients who were 48 cm or shorter No clear associations with survival related to birth weight or ponderal index. | Ponderal Index, gestational age |
| McCormack et al., 2003 Sweden | Fetal growth and subsequent risk of breast cancer: results from long term/ Cohort study follow up of Swedish cohort | 1915-1970 | 5,358 | 5,358 | Swedish Cancer Registry, The Uppsala Birth Cohort | Premenopausal women with a birth weight of <5.5 lbs (2,494.76 g) had a covariate-adjusted hazard ratio (HR) for breast cancer of 0.66 [95% confidence interval (CI) 0.47-0.93] compared with women born at 8.5 lbs (3,855.54 g) or above. Among postmenopausal women, no important association between the birth-weight and the incidence of breast cancer was detected (HR comparing women with a birth weight of 5.5 lbs or less with women with a birth-weight >8.5 lbs: 0.97; 95% CI 0.80-1.16) |
Gestational age, adult's height |
| Park et al., 2006 Poland | Intrauterine environment and breast cancer risk in a population- based / Case- control study in Poland | 2000-2003 | 2,385 | 4,888 | Population- based breast cancer case- control study in Warsaw and Lodz in Poland | Birth weights over 4,000 g were associated with a significantly increased risk of developing breast cancer compared to weights less than 2,500 g (OR 51.54 95% CI 1.08–2.19). | Maternal smoking and gestational age |
| Stavola et al., 2000, UK | Birth-weight, childhood growth and risk of breast cancer in a British cohort / Cohort study | 1971-1992 | 37 | 2,548 | The Medical Research Council National Survey of Health and Development | There was evidence of greater risk of breast cancer with greater birth-weight (rate ratio = 1.76 (95% CI: 0.92, 3.35) for birth-weight 3,500 g vs birth-weight < 3,500 g), which was more marked at pre-menopausal ages, RR = 2.31,95% CI: 0.93, 5.74). | Age, birth order, adult height, body mass index |
| Vatten et al., 2002, Norway | Birth weight as a predictor of breast cancer: a Case-Control study in Norway | 1910-1970 | 719 | 2,876 | Norwegian Cancer Registry | Birth weights in the highest quartile (3,730 g or more) were associated with 40% higher risk (odds ratio, 1.4, 95% confidence interval, 1.1 – 1.9) of breast cancer compared to birth weights in the lowest quartile (less than 3,090 g). | Mother's socioeconomic status |
| As expected, increasing age at first birth was associated with increasing risk of breast cancer, and there was a reduction in risk with increasing parity | |||||||
| Michels et al., 2006, USA | Longitudinal study of birth weight and the incidence of breast cancer in adulthood / Cohort Study | 1976-2001 | 2,969 | Health Study (NHS) and the Nurses' Health Study II (NHS II). | Premenopausal women with a birth weight of <5.5 lbs (2,494.76 g) had a covariate-adjusted hazard ratio (HR) for breast cancer of 0.66 [95% confidence interval (CI) 0.47–0.93] compared with women born at 8.5 lbs (3,855.54 g) or above | Age at menarche, adult height, family history of breast cancer | |
| META-ANALYSIS | |||||||
| Hodgson, 2004, USA | Birth weight, parental age, birth order and breast cancer risk in African- American and white women: a population- based / Case- Control study | May 1993-1996 | 196 | 363 | Birth records | Findings revealed that there was a weak inverse association between birth-weight in the highest tertile and breast cancer overall. Although associations varied by race. As high birth-weight was inversely associated with breast cancer among African-American women, and there was no association found with low birth-weight | Adjusted for maternal age, age, race, adult BMI, sampling fraction, and history of previous biopsy. |
| Innes 2004 USA | First pregnancy characteristics and subsequent breast cancer risk among young women A Case-control study | 1978-1995 | 2,522 | 10,052 | New York State birth and tumour registries | The data assessing birth weight and the risk of breast cancer. | Conditions on extreme prematurity, abruption of placentae, preeclampsia, perinatal factors, gestational hormones (particularly oestrogens). |
| Ahlgren, 2003, Denmark. | Birth weight and risk of breast cancer in a cohort of 106,504 women / Cohort study | 1968 - August | 2,334 | 106,405 | School health records | There was a significant positive association between birth-weight and breast cancer equivalent to a 9% increase in risk per 1,000 g increase in birth-weight | Adjusted for age and calendar period. An additional adjustment for parity and age at first birth did not indicate confounding. |
| Tius-Ernstoff, 2002, USA. | Early life factor in relation to breast cancer risk in postmenopausal women / A Case-control study | 1992-1994 | 1,716 | 1,886 | Telephone interview | A weak J-shaped relationship between breast cancer and birth- weight was observed; the increased risk was not statistically significant for either lower birth-weight or the high birth-weight. Overall results are consistent with previous studies and suggest that these early life factors have a modest influence on breast cancer risk in postmenopausal women | Covariates including other available early-life factors, parental smoking, religion, family history of breast cancer, parity, age at first full-term pregnancy, BMI at reference date, and age at menopause were considered, but the analysis provided no evidence of confounding, so final model adjusted only for age and state. |
| Innes, 2000, USA | Birth characteristics and subsequent risk for breast cancer in very young women / Case-control study | 1978-1995 | 484 | 2,870 | New York and New York City birth records | Birth-weight showed a J-shaped relation to breast cancer risk, which was said to be more with high birth-weight babies. In comparison to babies whose weight at birth range between 2,500-3,499 g, babies weighing 4,500 g and above were over 3 times more likely to develop breast cancer as a young adult. | Conditions on date of birth and maternal country of residence. Adjusted for gestational age, preeclampsia, abruption of placentae, multifetal gestation, birth rank, maternal age at birth, parental age at birth, and ethnicity. |
Of these 14 studies, 5 were case-control studies and 9 were cohort studies. However, only 5 out of the 14 included studies were eligible to be used for meta-analysis; these studies collected and presented outcome data and estimates of the association between risk of breast cancer and birth-weight using dichotomous comparisons. The number of the participants in the 5 studies used for meta-analysis varied from small (with 363 participants), to large (with 106,504 participants). The total number of 127,012 participants was extracted from across the 5 studies, of which 77,394 participants were in the sub-optimal birth- weight group, 46,317 participants were in the optimal birth-weight group, and 3,301 participants were in the above-optimal birth-weight group. The total number of breast cancer cases assessed was 7,239, of which 4,306 were in the sub-optimal group, 2,587 were in the optimal group, and 346 were in the above-optimal birth-weight group.
During quality assessment, using the STROBE Checklist, 6 of the 14 included studies were evaluated to be of high quality, while the remaining 8 studies were adjudged to be of medium quality. Primarily, the reasons for categorizing a study as lower quality were: variables and outcomes not clearly defined, study participants characteristics not provided, and study limitations not explained. The findings from the systematic review were presented under two themes: 1) Risk of breast cancer in sub-optimal birth-weight (3,500 g) and 2) Risk of breast cancer in above-optimal birth-weight (3,500-4,500 g) with optimal birth weight 3,500-4,500 g as the reference. The results from the meta-analysis were presented under the same themes.
Risk of breast cancer in sub-optimal birth-weight
Three (3) out of the 14 included studies documented an increased risk of breast cancer among women with sub-optimal birth-weight (3,500 g). The 3 studies were part of the 5 included in the meta-analysis, indicating that useful ranges of birth-weight data were presented. While the 3 studies were carried out in the United States, and used the same research design (case- control) 2 of the studies24,30 involved young women while the third one17 involved postmenopausal women. The 3 studies adjusted for various confounders such as maternal age, race, BMI, religion, parental smoking; only one of the studies17 adjusted for gestational age–the most potent confounder. Only one of the studies24 reported a weak relationship between low birth- weight and risk of developing breast cancer; however, this study involved postmenopausal women only. Seven (7) studies reported that risk of breast cancer decreased with lower birth-weight.
Risk of breast cancer in above-optimal birth-weight
Ten (10) out of the fourteen (14) included studies documented increased risk of breast cancer among women with above-optimal birth-weight (4,500 g). While the 10 studies were conducted in the US, UK and other European countries, different research designs (cohort and case-control study design) were used to assess the association. Five (5) out of the 10 studies17,20,31,32,33 reported that the association was stronger among premenopausal or young women; one study34 reported stronger association in postmenopausal women. In addition, all the 10 studies adjusted for potential confounders including gestational age, while 2 studies documented birth length and/or ponderal index as a better indicator to assess prenatal exposure and risk of developing breast cancer later in life.20,31 Interestingly, 2 out of the 13 studies reviewed, reported no significant relationship between birth-weight and the risk of developing breast cancer later in life, neither with low birth-weight nor high birth-weight.2,24 It must be noted that these two studies were conducted the US and Norway with the same research design – cohort study.
Meta-analysis of optimal birth-weight vs. sub-optimum birth-weight
Based on the five studies analysed, optimal birth weight (3,500-4,500 g) was found to be associated with increased risk of breast cancer when compared with sub-optimal birth-weight (3,500-4,500 g) (Figure 2). The pooled odd ratio (OR) estimate risk for breast cancer disease was 1.17 (95% CI 0.98, 1.39). However, there was an obvious heterogeneity between the studies included in the meta-analysis (Tau2=0.03, Chi2=28.93, df=4 (p<0.0000l); I2=86%), which informed our decision to use the random-effect model to compute the odds ratio. In addition, the forest plot showed that the diamond shape crossed the line of no effect (Figure 2), which implied that the sub-optimal birth-weight was not totally out of risk of breast cancer as reflected in the confidence interval (95% CI 0.98, 1.39). Finally, the overall effect of birth-weight (Z=1.74) on the risk of breast cancer was found to be not-significant (p=0.08).

- Forest plot of the risk of breast cancer for optimal birth-weight (3,500-4,500 g) versus sub-optimal birth-weight (<3,500 g). Studies were arranged according to their year of publication. The y-axis showed the range of 95% confidence interval (CI) for each study. Odds ratios (ORs) estimates of the risk of breast cancer for each study was indicated by the black diamond; the size of the square showed the statistical weight that each study contributed to the overall estimates of the square.
Meta-Analysis of optimal birth-weight vs. above-optimal birth-weight
Above-optimal birth-weight (>4,500 g) was found to be associated with increased risk of breast cancer when compared with optimal birth-weight (3,500-4,500 g) (Figure 3). The pooled odds ratios (OR) estimate risk for breast cancer diseases was 0.87 (95% CI 0.66, 1.15). There was an obvious heterogeneity between the studies included in the meta-analysis (Tau2=0.06; Chi2=11.13, df=4 (p=0.03); I2=64%), thus random-effect model was used. The overall effect of birth-weight (Z=0.98) on the risk of breast cancer was also not-significant (p=0.33) (Figure 3). From the forest plot, the diamond shape crossed the line of no effect, which indicated that the optimal birth-weight group was still at risk of breast cancer, as reflected in the odds ratio 0.87 (95% CI 0.66, 1.15).

- Forest plot on the risk of breast cancer for optimal birth-weight (3,500-4,500 g) verus above-optimal birth-weight (>4,500 g). Studies were arranged according to their year of publication. The y-axis showed the range of 95% confidence interval (CI) for each study. Odds ratios (ORs) estimates of the risk of breast cancer for each study was indicated by the black diamond; the size of the square showed the statistical weight that each study contributed to the overall estimates of the square.
Discussion
This systematic review study has provided a more robust and broader insight into the relationship between birth-weight and risk of breast cancer later in life, by highlighting the quality, distribution, and the characteristics of studies. Only a few studies were found to be eligible based on the eligibility criteria that was used. The findings from the review showed that there is a clear relationship between high birth-weight (>4,500 g) and risk of breast cancer; this relationship was found to be more pronounced among premenopausal women by most of the studies included in the review.17,20,31,31,33 In addition, the meta-analysis study – which was based on 127,012 participants drawn from the 5 eligible studies - showed that the risk of breast cancer increased with increasing birth-weight, such that participants with birth-weights >4,500 g were found to be at a greater risk of breast cancer.
On the other hand, our findings, both from the systematic review and meta-analysis, showed that participants with optimal birth-weight (3,500-4,500 g) are not at a lesser risk of developing breast cancer as hypothesized, while participants with sub-optimal birth-weights (<3,500 g) were not at a higher risk. Therefore, the hypothesized J-shaped relationship was not observed in this study. Within the meta-analysis, significantly high heterogeneity among the studies was observed, which is likely due to the various methodologies used by the included studies; however, this was addressed in the analysis by using the random-effect model to estimate the odd ratios.27 In addition, none of the estimated risk were significant (p<0.05); however, findings from the systematic review revealed that risks of breast cancer in association with birth-weight is more pronounced in premenopausal women, below 50 years in age. This might have affected the findings in the meta-analysis as the data from the included studies could not be disaggregated by menopausal status or age.
Meanwhile, the findings from this study are consistent with other bodies of literature– which have indicated that breast cancer risk increases with high birth-weight.2,18,27,33,35,36,37,38,39 A large meta-analysis study,19 involving 2,334 breast cancer cases and 106,504 participants, found a significantly positive association between birth-weight and breast cancer risk, such that a 1,000g rise in birth-weight was estimated to increase the risk of breast cancer by 9% (OR=9, 95% CI= 2% to 17%). Another meta-analysis study17 pointed that the evidence from available data strongly suggested a positive relationship between birth-weight >4,000 g and increased risk of breast cancer later in life.
The biological mechanisms that underpin this relationship have been under investigation; strongest among them, is the developmental origin of health and diseases (DOHaD) theory postulated by Baker.13,40,15 According to this theory, it is believed that adverse intrauterine exposure of the fetus, which seems to have an overwhelming impact on birth size, can result in a permanent change in the physiology and metabolism of the fetus, thus, increasing the susceptibility to diseases later in life.41,42,43 There is a growing body of evidence that the risk of breast cancer later in life is largely influenced by intrauterine exposure – which is usually assessed based on birth-weight as a surrogate indicator. In animal experiments,34,44,45 exposure to increased maternal oestrogen levels- a hormonal exposure that has been linked to birth-weight,30,46 during fetal and early postnatal development, has been found to make marked changes to fetal mammary development. This in turn may increase the risk of breast cancer in adulthood. In addition, breast cancer risk in association with high birth-weight may be confined to oestrogen receptor-positive (ER+) tumors and progesterone receptor positive (PR+) tumors thus stressing on the potential mechanistic role of the sex steroid hormonal pathway.
On the other hand, findings from a few studies also indicated that there is a weak relationship between birth-weight and the risk of breast cancer.24,30 A study conducted in Sweden using birth records,23 reported a positive associated risk of breast cancer in low birth-weight and high birth- weight participants. In another study,23 the same author could not confirm an association between birth-weight and breast cancer risk, while using the birth records information used in the first study plus the records of four additional hospitals.
Limitations
There is a need to interpret the findings from this systematic review and meta-analysis carefully, due to some limitations encountered during the study. There was a significant level of heterogeneity among the studies included in the meta-analysis. This was addressed by using a random effect model to compute the odds ratio. In addition, due to the small number of studies involved in the meta-analysis, we could not conduct meta-regression and publication bias. Furthermore, only English Language studies were included, and all the studies were conducted in Europe and in the US; hence, this possibly affected the generalizability of the study.
Conclusion and Implications for Translation
A total of 14 studies for the systematic review and data derived from 127,012 participants across the selected studies included in the meta-analysis provided a more robust and clearer evidence on the relationship between birth-weight and risk of breast cancer later in life. Using a systematic review methodology, which is usually the highest in hierarchy of evidence in medical research, there is a clear relationship between high birth-weight and risk of breast cancer; this relationship was found to be more pronounced among premenopausal women.
In developed countries, which had the highest incidence rate of breast cancer, prevention programs through health education and early diagnosis strategies targeted at this group might be a promising strategy to tackle its associated burden. This is also important in developing countries, where 58% of breast cancer-associated deaths occur, but patients have the lowest survival rate due to late diagnosis of most cases of breast cancer. In addition, further research is needed to understand the underlying factors between the risk of breast cancer and high birth-weight, especially among premenopausal women.
Compliance with Ethical Standards
Ethics Approval:
This study is based on analysis of existing data.
Conflicts of Interest:
There is no conflict of interest in the research paper.
Financial Disclosure:
Nothing to declare.
Disclaimer:
None.
Acknowledgment:
Dr. Denise Belleigham-Young for introducing meta-analysis and providing technical assistance at different stages during the study.
Funding:
The authors did not receive any funding for this study.
References
- Epidemiology of breast cancer. Lancet Oncol. 2001;2(3):133-140.
- [CrossRef] [PubMed] [Google Scholar]
- Birth weight and breast cancer risk. Br J Cancer. 2006;94(11):1734-1737.
- [CrossRef] [PubMed] [Google Scholar]
- Birth weight and other prenatal factors and risk of breast cancer in Asian-Americans. Breast Cancer Res Treat. 2011;130(3):917-925.
- [CrossRef] [PubMed] [Google Scholar]
- Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):359-386.
- [CrossRef] [PubMed] [Google Scholar]
- ABC of Breast Diseases 2006
- The risk of infectious diseases associated with birth-weight: a systematic review and meta-analysis. Int J Sci Eng Res. 2017;8(5):1671-1679.
- [Google Scholar]
- The impact of birth weight on adult minor illness: a study on a sub-clinical population. Rev Bras Crescimento Desenvolv Hum. 2013;23(1):11-17.
- [CrossRef] [Google Scholar]
- et al. Birthweight and mortality in adulthood: a systematic review and meta-analysis. Int J Epidemiol. 2011;40(3):647-661.
- [CrossRef] [PubMed] [Google Scholar]
- Birth weight and metabolic syndrome in adults: meta-analysis. Rev Saúde Pública. 2008;42(1):10-8.
- [CrossRef] [Google Scholar]
- Birth Weight and Long-Term Overweight Risk: Systematic Review and a Meta-Analysis Including 643,902 Persons from 66 Studies and 26 Countries Globally. PLoS ONE. 2012;7(10):1-10.
- [CrossRef] [PubMed] [Google Scholar]
- More than genes: the advanced fetal programming hypothesis. J Reprod Immunol 2014:104-105. 8-11
- [CrossRef] [PubMed] [Google Scholar]
- Osmond C. Fetal origins of adult disease: strength of effects and biological basis. Int J Epidemiol. 2002;31(6):1235-1239.
- [CrossRef] [PubMed] [Google Scholar]
- Birthweight, childhood growth and risk of breast cancer in a British cohort. Br J Cancer. 2000;83(7):964-968.
- [CrossRef] [PubMed] [Google Scholar]
- Willett WC. Longitudinal study of birth weight and the incidence of breast cancer in adulthood. Carcinogenesis. 2006;27(12):2464-2468.
- [CrossRef] [PubMed] [Google Scholar]
- Birth weight as a predictor of breast cancer: a case- control study in Norway. Br J Cancer. 2002;86(1):89-91.
- [CrossRef] [PubMed] [Google Scholar]
- Birth weight and risk of breast cancer in a cohort of 106,504 women. Int J Cancer. 2003;107(6):997-1000.
- [CrossRef] [PubMed] [Google Scholar]
- Fetal growth and subsequent risk of breast cancer: results from long term follow up of Swedish cohort. BMJ. 2003;326(7383):248-253.
- [CrossRef] [PubMed] [Google Scholar]
- Birth characteristics of premenopausal women with breast cancer. Br J Cancer. 1988;57(4):437-439.
- [CrossRef] [PubMed] [Google Scholar]
- Intrauterine environment and breast cancer risk in women: a population-based study. J Natl Cancer Inst. 1997;89(1):71-76.
- [CrossRef] [PubMed] [Google Scholar]
- Early life factors in relation to breast cancer risk in postmenopausal women. Cancer Epidemiol Biomarkaer Prev. 2002;11(2):207-210.
- [Google Scholar]
- Maternal factors and breast cancer risk among young women. Paediatr Perinat Epidemiol. 1998;12:397-407.
- [CrossRef] [PubMed] [Google Scholar]
- Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. J Am Med Assoc. 2000;283(15):2008-2012.
- [CrossRef] [PubMed] [Google Scholar]
- Systematic Review in Healthcare- Meta-analysis in Context 2011
- Strengthening the reporting of observational studies in epidemiology: STROBE checklists. STROBE (accessed )
- [Google Scholar]
- Review Manager (accessed )
- Birth weight, parental age, birth order and breast cancer risk in African-American and white women: a population- based case-control study. Breast Cancer Res. 2004;6(6):R656-667.
- [CrossRef] [PubMed] [Google Scholar]
- Birth length and weight as predictors of breast cancer prognosis. BMC Cancer. 2010;10:115.
- [CrossRef] [PubMed] [Google Scholar]
- Preterm birth, birth weight, and subsequent risk of female breast cancer. Br J Cancer. 2003;89(9):1664-1666.
- [CrossRef] [PubMed] [Google Scholar]
- Birth weight, childhood growth and risk of breast cancer in a British cohort. Br J Cancer. 2000;83(7):964-968.
- [CrossRef] [Google Scholar]
- Intrauterine environment and breast cancer risk in a population-based case-control study in Poland. Int J Cancer. 2006;119(9):2136-2141.
- [CrossRef] [PubMed] [Google Scholar]
- Birth charateristics and breast cancer risk: a study among like-sexed twins. Int J Cancer. 2001;91(2):248-25I.
- [CrossRef] [PubMed] [Google Scholar]
- Growth Patterns and the Risk of Breast Cancer in Women. N Engl J Med. 2004;351(16):1619-1626.
- [CrossRef] [PubMed] [Google Scholar]
- Birth weight and risk of early- onset breast cancer (Denmark) Cancer Causes Control. 2003;14(1):61-4.
- [CrossRef] [PubMed] [Google Scholar]
- Birth weight-breast cancer revisited: is the association confounded by familial factors? Cancer Epidemiol Biomarkers Prev. 2009;18(9):2447-52.
- [CrossRef] [PubMed] [Google Scholar]
- Birth size and breast cancer risk: re-analysis of individual participant data from 32 studies. PLoS Med. 2008;5(9):e193.
- [CrossRef] [PubMed] [Google Scholar]
- New insight into ending chronic disease 1995
- [CrossRef]
- Developmental origins of health and disease: reducing the burden of chronic disease in the next generation. Genome Med. 2010;2(2):14.
- [CrossRef] [PubMed] [Google Scholar]
- Developmental origins of disease paradigm: a mechanistic and evolutionary perspective. Pediatr Res. 2004;56(3):311-317.
- [CrossRef] [PubMed] [Google Scholar]
- The Fetal Matrix: Evolution, Development, and Disease 2005
- [CrossRef]
- Menopausal estrogen and estrogen- progestin replacement therapy and breast cancer risk. JAMA. 2000;283(4):485-91.
- [CrossRef] [PubMed] [Google Scholar]
- Fetal origins of breast cancer. Trends Endocrinol Metab. 2006;17(9):340-348.
- [CrossRef] [PubMed] [Google Scholar]
- Promoting Optimal Fetal Development Report of a Technical Consultation 2003 (accessed )





